4714
Accelerated Time Resolved Phase Contrast Cerebral MRA to Evaluate Pulse Wave Velocity1Department of Computer Science and Information Engineering, National Cheng Kung University, Tainan, Taiwan, 2Institute of Medical Informatics, National Cheng Kung University, Tainan, Taiwan, 3Department of Automatic Control Engineering, Feng-Chia University, Taichung, Taiwan, 4Graduate Institute of Oncology, National Taiwan University, Taipei, Taiwan, 5Graduate Institute of Biomedical Electronics and Bioinformatics, National Taiwan University, Taipei, Taiwan, 6Department of Biomedical Engineering and Environmental Sciences, National Tsing Hua University, Hsinchu, Taiwan, 7Department of Electrical Engineering, National Sun Yat Sen University, Kaohsiung, Taiwan, 8Department of Electrical Engineering, National Taiwan University, Taipei, Taiwan, 9Department of Electrical Engineering, National Taiwan University of Science and Technology, Taipei, Taiwan
Synopsis
Time-resolved PCMRI has been applied to measure pulse wave velocity (PWV) for the assessment of aortic stiffness. However, longer scan time hinders its practice to achieve a high spatial and temporal resolution scan, especially required for the arteries inside of the head and neck. In the present work, an accelerated Time-resolved PCMRA was implemented to shorten scan time. The reconstruction combines temporal strategy and self-reference information to retain reasonable imaging quality. The results suggest that the proposed method differentiate PWV delay time from Common-Carotid artery to Middle-Cerebral arteries via Internal-Carotid artery with good quality within a 10-minute scan.
Purpose
Time resolved phase contrast MRI (Time-resolved PCMRI)1 has been extensively used to evaluate vascular function such as 4D flow2. The potential of this technique includes providing dynamic physiological information and morphology of the imaged vessels. While Time-resolved PCMRI has been successful in monitoring dynamics of cardiovascular system, its application to cerebral vessels as Time-resolved PCMRA and Pulse wave velocity (PWV) imaging is still challenging due to the requirement of high temporal and spatial resolution and the inherited long scan time. Although ultrasound also provides 2D illustration of PWV3, the imaging region is limited to the anatomy where ultrasonic wave can go through. Henceforth, an MR-based alternative should be favorable when deeper brain regions are of interest. The present work aims at developing an acceleration strategy for Time-resolved PCMRA covering from carotid arteries to its downstreams for PWV evaluation. Current implementation involves the two strategies, MUSE4 and hybrid SENSE5 in a hope to retain morphological and dynamic information at good quality within short scan time.Methods
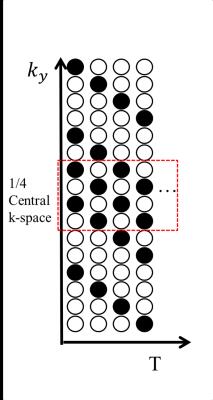
Four healthy subjects were recruited and scanned following informed consent, using an IRB-approved protocol on a 3.0T scanner (GE Discovery MR750). After scout scans to locate the orientation of carotid arteries, the proposed fast Time-Resolved PC MRI was performed with eight 7-mm slices (FOV = 26cm within a 256x256 matrix) to encompass carotid arteries and its downstream branches. The other imaging parameters are as follows: TR=5.0ms, TE=2.8ms , View per segment=1, and Venc=180cm/s along three orthogonal directions leading to temporal resolution equal to 20ms. A variable density sampling function (Fig. 1) covering the central quarter of k-space at two-fold acceleration and the rest at four-fold acceleration was used. The total scan time for a single slice required 80 cardiac cycles. Despite heart rate variability for each subject and different heart rates among subjects, the scans were all done within 10 minutes. Photoplethysmogram(PPG) was used to monitor pulses for cardiac gating and for gradient encoding synchronization to achieve fully gating reconstruction. The data without velocity encoding were firstly averaged to remove artifacts for coil sensitivity estimation. The acquired data were processed by a two pass reconstruction to improve SNR and to retain temporal accuracy as suggested in the reference algorithms4-6. The first-pass reconstruction was performed using SENSE on all the acquired data with prospective cardiac gating. A spatial low-pass filter was applied after the reconstruction to improve SNR and to serve as the regularization information in the second-pass reconstruction. Subsequently, the original undersampled data were retrospectively regridded in terms of cardiac phase and processed by the algorithm expressed by:$$ \widehat{\rho}_{y,f,\overrightarrow{v}}=argmin_{\rho_{y,f,\overrightarrow{v}}}||s_{k,t,\overrightarrow{v}}-E_{k,t} \rho_{y,f,\overrightarrow{v}}||^2_2 - \lambda||M^{-1}_{y,f,\overrightarrow{v}}\rho_{y,f,\overrightarrow{v}}||^2_2 $$, where $$$E_{k,t}$$$ represents the encoding matrix including Fourier transformation from k-t space, the sampling trajectory, the density compensation and the coil sensitivity, $$$M^{-1}_{y,f,\overrightarrow{v}}$$$ stands for the regularization information from the filtered first-pass reconstruction with its weight $$$\lambda$$$ to minimize noise amplification, $$$\rho_{y,f,\overrightarrow{v}}$$$ denotes the final reconstruction and $$$s_{k,t,\overrightarrow{v}}$$$ is the input rawdata. The number of cardiac phases in retrospectively-gating reconstruction was set to be 80. For the visualization purpose and the analysis of PWV, $$$\rho_{y,f,\overrightarrow{v}}$$$ is then transformed back to time domain to calculate flow speed and direction multiplied by the magnitude mask to enhance temporal vascular signals.Results
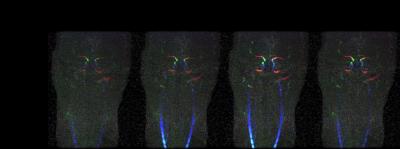
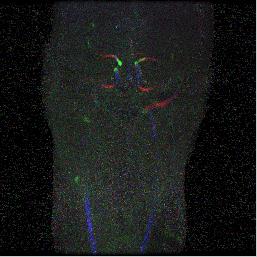
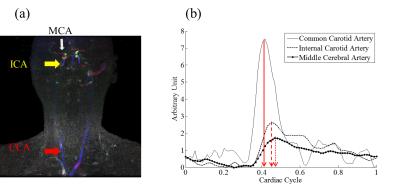
Fig.2 shows the temporal flow speed changes from subject 1. Enhancement can be found starting from the common carotid arteries (CCA) followed by the internal carotid arteries (ICA) then to the middle cerebral arteries (MCA). The animated version of the full time series is shown in Fig. 3 on the on-line version. Figure 4a shows a spatial-temporal Maximum Intensity Projection (MIP) result using data from subject 2. Temporal signals from the three ROIs, CCA, ICA and MCA, are shown in Figure 4b. Significant delay among different vascular segments can be noticed and due to high temporal resolution, delay time from the adjacent ROIs of ICA and MCA could still be resolved.Discussions and Conclusion
The results suggest that to resolve small vessels and the latency of pulsatile flow in the cerebral arteries required high spatial and temporal resolution. If no acceleration strategy is involved in the sequence, it will take 256 cardiac cycles to achieve similar spatial and temporal resolution for scanning one slices, roughly around 4 minutes. The volumetric scan using proposed method has brought the scan time down to 10 minute. According to Figs 2 and 4, not only carotid arteries and its downstreams but also the vertebral arteries and the basilar arteries can be enhanced. Thus the present strategy could have the potential to help monitor cerebral PWV as a risk factor of cerebral vascular stiffness7.Acknowledgements
Acknowledgements: Support is acknowledged from MOST grants:105-2314-B-006 -044 –MY2, 105-2314-B-007-003, and 105-2221-E-035 -049 -MY2 and Mind Research and Imaging Center in National Cheng-Kung University.References
1. Markl, M., et al., Time-resolved three-dimensional phase-contrast MRI. J Magn Reson Imaging, 2003. 17(4): p. 499-506.
2. Markl, M., et al., 4D flow MRI. J Magn Reson Imaging, 2012. 36(5): p. 1015-36.
3. Luo, J., R.X. Li, and E.E. Konofagou, Pulse wave imaging of the human carotid artery: an in vivo feasibility study. IEEE Trans Ultrason Ferroelectr Freq Control, 2012. 59(1): p. 174-81.
4. Chen, N.K., et al., A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). Neuroimage, 2013. 72: p. 41-7.
5. Chao, T.C., et al., A 2D MTF approach to evaluate and guide dynamic imaging developments. Magn Reson Med, 2010. 63(2): p. 407-18.
6. Chao, T.C., et al., Fast diffusion imaging with high angular resolution. Magn Reson Med, 2016.
7. Willum-Hansen, T., et al., Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation, 2006. 113(5): p. 664-70.
Figures