4712
T2*-weighted imaging of the mouse neurovasculature without contrast agent1Centre d'imagerie moléculaire de Sherbrooke, department of Médecine nucléaire et radiobiologie, Université de Sherbrooke, Sherbrooke, QC, Canada
Synopsis
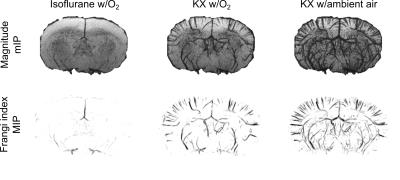
While standard isoflurane anesthesia does not easily allow visualization of small vessels in mice using T2*-weighted imaging, ketamine/xylazine anesthesia enables the visualization of an impressive fraction of the vasculature, without the need of an external contrast agent. Visualization can be further modulated by modifying the breathing gas.
Introduction
Imaging neurovasculature noninvasively in mouse models is an important step toward a better understanding of both pathological and normal conditions of the brain. T2*-weighted imaging is already a method of choice to image small vessels containing molecules that perturb the magnetic field such as deoxyhemoglobin or an extrinsic contrast agent. The long-range spin dephasing induced by these molecules reveals vessels smaller than the acquisition resolution. In rats, vein imaging based on deoxyhemoglobin and a T2*-weighted sequence has already provided valuable information in a stroke model1. In mice, however, T2*-weighted imaging of the brain vasculature was always preceded by an injection of superparamagnetic iron oxide particles. Pre-injection images revealed only a very limited fraction of the vessels, insufficient for further analysis2–4. On the other hand, it has already been shown in rats that changing the type of anesthesia has a clear impact on the T2*-weighted contrast5. Here, we show that while standard isoflurane anesthesia does not easily allow visualization of small vessels in the mouse brain using T2*-weighted imaging, ketamine/xylazine (KX) anesthesia enables the visualization of an impressive fraction of the vasculature, without the need of an external contrast agent.Material and methods
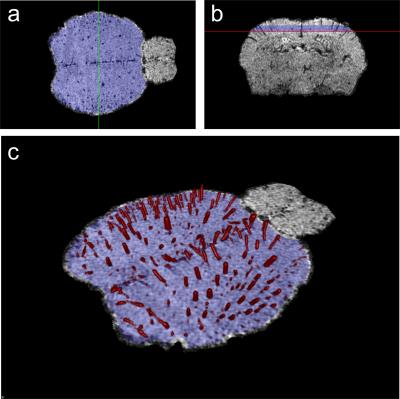
A total of 29 data sets were acquired from 6 BALB/c mice with a 210-mm small animal 7T scanner (Varian Inc., Palo Alto, CA) and a dedicated mouse head-coil (RAPID MR International, OH). Mouse brains were imaged with a 3D gradient echo sequence with TR=50 ms, TE=25 ms, flip angle=15°, resolution=78×78×104 mm3, field of view=20×15×10 mm3 or 20×20×10 mm3, 2 averages, flow compensation in all direction and phase encoding rewind. Mice were imaged under KX anesthesia (intraperitoneal injection of 87 mg of ketamine and 13 mg of xylazine per kg of bodyweight) while breathing O2 provided at 1.5 liter/minute (N=4) or ambient air (N=7). The procedure was repeated on a different day under isoflurane anesthesia (1.5% isoflurane in O2 flowing at 1.5 liter/minute; N=2 with the MR parameters described above and N=16 with an array of TR, TE, flip angle and spatial resolution). We applied the following image processing steps: 1) intensity bias correction using N4ITK algorithm6; 2) brain segmentation with the brain extraction tool7 and 3) vessel segmentation with histogram-based thresholding of the Frangi index8. Vascular density was computed in a region of interest (ROI) located in the superior cortex.Results
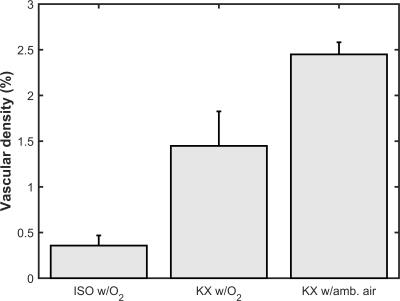
As seen in figure 1, isoflurane anesthesia only allows visualization of main vessels. Vessels conspicuity was not significantly improved by acquiring data with an array of TR, TE, flip angle and spatial resolution (data not shown). With KX anesthesia, there is a dramatic increase in the number of vessels that can be visualized. This increase is even more pronounced with the inhalation of ambient air (figure 1). Image analysis allowed visualization and quantification of the vascular density in a ROI in the superior cortex (figure 2). The vascular density in this ROI reflects the important effect of anesthesia conditions on vessels conspicuity (figure 3).Discussion
T2*-weighted imaging of vasculature without extrinsic contrast agent mainly relies on the paramagnetism of deoxyhemoglobin9. Blood oxygenation is therefore most likely to be one of the main factors explaining vessel contrast differences between anesthesia types, with isoflurane causing higher oxygenation than KX, and O2 breathing causing higher oxygenation than ambient air breathing. This hypothesis is coherent with studies on cerebral blood flow (CBF) and cerebral metabolic rate of O2 (CMRO2) in rodents. Observations indeed indicated higher CBF with isoflurane than with KX10,11, while studies in mice suggested similar or lower CMRO2 for isoflurane than for KX12–14. The observed dependence of vessels conspicuity with anesthesia type is also in line with results obtained in the rat5. While no toxic effect was noticed over the full length of the experiment, KX anesthesia, especially with ambient air breathing, may cause hypoxia. The next step involves measuring the blood gas and oxygenation levels as a function of the anesthesia conditions.Conclusions
The results show the effect of the anesthesia conditions on the visualization of mouse neurovasculature. Unlike standard isoflurane anesthesia, KX anesthesia enables visualization of small vessels in the mouse brain using T2*-weighted imaging without the need of an extrinsic contrast agent. Vessels conspicuity can be further modulated by modifying the breathing gas. Understanding the neurovascular coupling in fMRI or developing novel drugs acting on vascularization are some of the many fields that can benefit from a more accurate imaging of the mouse neurovasculature.Acknowledgements
Jérémie Fouquet is supported by a NSERC scholarship. Martin Lepage is member of the FRQS-funded Centre de recherche du CHUS. We thank Mélanie Archambault and Dina Sikpa for animal handling and preparation, as well as Matthieu Dumont for the help provided for image treatment and analysis.References
1. Huang C-H, Chen C-C V., Siow T-Y, et al. High-Resolution Structural and Functional Assessments of Cerebral Microvasculature Using 3D Gas ΔR2*-mMRA. Sen U, ed. PLoS One. 2013;8(11):e78186.
2. Klohs J, Baltes C, Princz-Kranz F, et al. Contrast-Enhanced Magnetic Resonance Microangiography Reveals Remodeling of the Cerebral Microvasculature in Transgenic ArcA Mice. J. Neurosci. 2012;32(5):1705–1713.
3. Lin C-Y, Hsu Y-H, Lin M-H, et al. Neurovascular abnormalities in humans and mice with Huntington’s disease. Exp. Neurol. 2013;250:20–30.
4. Hamans BC, Barth M, Leenders WP, et al. Contrast enhanced susceptibility weighted imaging (CE-SWI) of the mouse brain using ultrasmall superparamagnetic ironoxide particles (USPIO). Z. Med. Phys. 2006;16(4):269–274.
5. Ciobanu L, Reynaud O, Uhrig L, et al. Effects of anesthetic agents on brain blood oxygenation level revealed with ultra-high field MRI. Chang AYW, ed. PLoS One. 2012;7(3):e32645.
6. Tustison NJ, Avants BB, Cook PA, et al. N4ITK: Improved N3 bias correction. IEEE Trans. Med. Imaging. 2010;29(6):1310–1320.
7. Smith SM. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17(3):143–55.
8. Frangi A, Niessen W. Multiscale vessel enhancement filtering. Lect. Notes Comput. Sci. - Lect. Notes Med. Image Comput. Comput. Interv. -- MICCAI’98. 1998;1496:130–137.
9. Reichenbach JR, Haacke EM. High-resolution BOLD venographic imaging: a window into brain function. NMR Biomed. 2001;14(7–8):453–67.
10. Lei H, Grinberg O, Nwaigwe CI, et al. The effects of ketamine-xylazine anesthesia on cerebral blood flow and oxygenation observed using nuclear magnetic resonance perfusion imaging and electron paramagnetic resonance oximetry. Brain Res. 2001;913(2):174–179.
11. Ku T, Choi C. Noninvasive Optical Measurement of Cerebral Blood Flow in Mice Using Molecular Dynamics Analysis of Indocyanine Green. PLoS One. 2012;7(10):1–10.
12. Chong SP, Merkle CW, Leahy C, et al. Cerebral metabolic rate of oxygen (CMRO2) assessed by combined Doppler and spectroscopic OCT. Biomed. Opt. Express. 2015;6(10):3941.
13. Zhu X-H, Chen JM, Tu T-W, et al. Simultaneous and noninvasive imaging of cerebral oxygen metabolic rate, blood flow and oxygen extraction fraction in stroke mice O MRS imaging CMRO 2 CBF OEF Stroke. Neuroimage. 2013;64:437–447.
14. Cui W, Zhu X-H, Vollmers ML, et al. Non-invasive measurement of cerebral oxygen metabolism in the mouse brain by ultra-high field (17)O MR spectroscopy. J. Cereb. Blood Flow Metab. 2013;33(12):1846–9.
Figures