4706
Intracranial Vessel Analysis (IVA): A Toolkit for Semi-Automatic Morphological Quantification of Intracranial Atherosclerotic Plaque1Biomedical Imaging Research Institute, Cedars Sinai Medical Center, Los Angeles, CA, United States, 2Electrical and Computer Engineering, University of Michigan, MI, United States, 3Department of Radiology, Xuanwu Hospital, Beijing, People's Republic of China
Synopsis
Intracranial atherosclerosis is a disease in which a sticky substance called plaque builds up inside the arteries. In practice, plaque analysis based on high resolution MRI is largely conducted manually by neuroradiologists with qualitative results. In this study, we propose a framework for intracranial vessel analysis (IVA), for semi-automatic morphological quantification of intracranial atherosclerotic plaque. Briefly, the framework includes functions of vessel path tracking, 3D MPR, vessel wall segmentation, measurement calculation, and report generation, with minimal user intervention required. Experiments show that plaque existence and location could be easily determined from the resulting vessel wall measures.
Purpose
Intracranial atherosclerosis disease (ICAD) is one of the main causes for cerebrovascular events, accounting for 8% to 10% of strokes in North America and even higher in Asia 1. High resolution magnetic resonance imaging (HR-MRI), particularly using 3D turbo spin-echo (TSE) with variable refocusing flip angles, has widely been used as a research tool to assess intracranial vessel wall and ICAD 2,3,4. However, plaque analysis based on HR-MRI currently relies on image review by neuroradiologists, which is effort demanding and experience dependent. More importantly, results are typically qualitative rather than quantitative. This is perhaps one of reasons precluding wide application of HR-MRI in clinics. In this study, we propose a framework for intracranial vessel analysis (IVA), for semi-automatic morphological quantification of intracranial atherosclerotic plaque.Methods
The IVA framework was built based on 3D HR-MRI images acquired using our recently developed T1-weighted whole-brain vessel wall imaging method, namely inversion-recovery prepared 3D TSE. One of the advantage of this method is that it has superior signal suppression of cerebrospinal fluid (CSF) and blood, and vessel wall is of high contrast and readily to be localized.
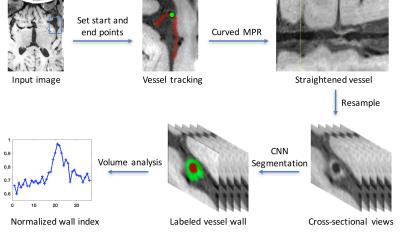
The flow chart of the IVA framework is illustrated in Fig. 1. A 3D HR-MRI dataset is first imported as the input image on which the user is able to pick the interested vessel and manually place the start and end points. An automatic histogram based method is designed to track the vessel path between these two points. Briefly, we first apply histogram equalization in the local neighborhood of the given points using multiple local window size to emphasize local contrast. A Bayesian classifier with Gaussian mixture models is used to classify the vessel lumen region. The gradient in the lumen region provides the direction of vessel and its cross-sectional view is used to find the center of vessel. This process is repeated until a shortest path is found as the vessel centerline between the start and end points.
Next, 3D curved multiplanar reformation (MPR) is applied to straighten the vessel, which allows the user to readily follow the course of a tortuous vessel for a long distance despite its changing direction. Cross-sectional 2D slices of the vessel segment are obtained through resampling and then undergo vessel wall segmentation algorithm. Convolution neural network (CNN) is employed here as a recently emerged powerful machine learning method for semantic segmentation 5. In particular, each of the 2D slices is considered as inputs, and will go through the convolution process that is composed of 9 convolution layers and 3 pooling layers, resulting in hierarchical extraction of low- and high-order convolutional features. These features will then undergo a deconvolution process to produce an upsampled segmentation result that has the same dimension as the input image.
From the segmentation results, we define normalized wall index (NWI) as the wall area divided by the summation of wall area and lumen area. NWI ranges from 0 to 1, where a higher value means a higher percentage of wall area which more likely indicates the existence of a plaque.
Results
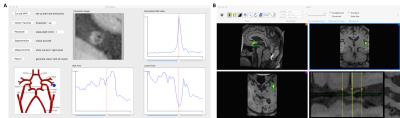
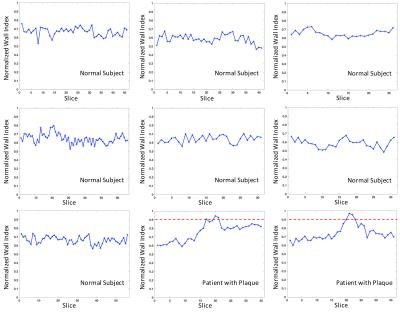
We implemented the framework as a plugin toolkit based on an open source software OsiriX 6, which runs Cocoa environments in MacOSX. Fig. 2A shows the interface of our toolkit, and the curved MPR calls the interface of Fig. 2B, where the user can define the start and end points. The step length defines the thickness of 2D slices. After segmentation, manual checking and editing is permitted if needed. NWI is then computed and volume plots are shown in the right panel. A schematic map of brain vessels is included where a blue marker could be manually placed to indicate the investigated area, which would be used in the automatic report generation. Fig. 3 shows the NWI curves of 7 normal subjects and 2 patients with plaques, where a 0.9 threshold of NWI can easily separate the patients from normal subjects.Discussion
We have outlined the main function and user interface of the proposed IVA toolkit. It allows path finding and automatic segmentation with minimal user intervention required, and will save user’s time and efforts in processing intracranial images for plaque quantification and report generation. It is still an ongoing work and we will rigorously evaluate the accuracy and reliability of the framework and toolkit.Acknowledgements
This work was supported in part by American Heart Association (15SDG25710441), and National Institutes of Health (NHLBI 2R01HL096119).References
1 Turan TN, Derdeyn CP, Fiorella D, Chimowitz MI. Treatment of atherosclerotic intracranial arterial stenosis. Stroke 2009; 40(6):2257-2261.
2 Qiao Y, Steinman DA, Qin Q, Etesami M, Schär M, Astor BC, Wasserman BA. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 Tesla. Journal of Magnetic Resonance Imaging 2011; 34(1):22-30.
3 van der Kolk AG, Hendrikse J, Zwanenburg JJ, Visser F, Luijten PR. Clinical applications of 7T MRI in the brain. European journal of radiology 2013; 82(5):708-18.
4 Fan Z, Yang Q, Deng Z, Li Y, Bi X, Song S, Li D. Whole brain intracranial vessel wall imaging at 3 Tesla using cerebrospinal fluid attenuated T1 weighted 3D turbo spin echo. Magnetic resonance in medicine 2016; in press.
5 Long J, Shelhamer E, Darrell T. Fully convolutional networks for semantic segmentation. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition 2015; 3431-3440.
6 Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. Journal of digital imaging 2004; 17(3):205-216.
Figures