4704
Is the length of the white matter fiber bundles underlying the thalamo-cortical loop associated with sleep spindles? – a preliminary study1Center for advanced research in sleep medicine, Hôpital du Sacré-Coeur de Montréal, Montreal, QC, Canada, 2Department of Psychology, University of Montreal, Montreal, QC, Canada, 3Research Center, Institut universitaire de gériatrie de Montréal, Montreal, QC, Canada, 4Sherbrooke connectivity imaging lab, Computer science Department, University of Sherbrooke, Sherbrooke, QC, Canada
Synopsis
Sleep spindles, an EEG manifestation generated by the thalamo-cortical loop and implicated in sleep-dependent learning were recently associated to voxel-based metrics of brain white matter. Thus, we aimed to investigate if specific bundles of streamlines underlying the thalamo-cortical loop will be associated to sleep spindles variables in twenty-five young subjects. Our study showed that the median fiber length of streamlines connecting the thalamus to the anterior and middle part of the superior frontal gyrus significantly predicted sleep spindles amplitude and frequency measured on frontal and central electrodes.
Introduction
Sleep spindles are electroencephalographic manifestations occurring during non-rapid eye movement sleep which are characterized by bursts of oscillatory brain activity with a frequency varying between 12 and 16 Hz. Studies showed that sleep spindles play a significant role in sleep-related cognitive processes such as declarative and procedural memory consolidation as well as in more global cognitive abilities such as the intelligence.1-4 The generation of sleep spindles imply a complex activation of the thalamo-cortico-thalamic loop.5 The literature suggests that sleep spindles reflect dynamic connectivity in-between neuronal networks in the brain but also more stable white matter networks as demonstrated by a recent study that showed in young subjects an association between sleep spindles power and white matter microstructure using tract-based spatial statistics, a voxel-based approach.6 Thus, this study aimed at investigating whether sleep spindles variables were associated to characteristics of the thalamo-cortical loop using a streamline-based and bundle specific approach measuring streamline length between the thalamus and the frontal cortex.Methods
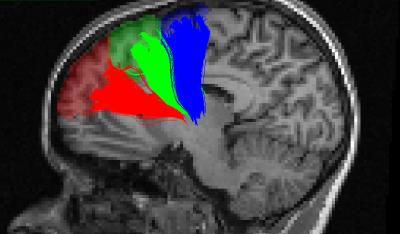
Twenty-five subjects (20-30 years, 11 females) underwent a whole-night of polysomnographic recording and a 3T MRI acquisition including a diffusion sequence (EPI, 64 gradient directions, b-value 700 s/mm2) and a structural T1 weighted image. Sleep spindles were automatically detected on artefact-free non-rapid eye movement sleep using a previously published detection algorithm on F3, F4, C3, and C4 electrodes referred to linked-earlobes7. Sleep spindles detected in N2 sleep stage were analysed. Sleep spindles amplitude (μV) and frequency (Hz) were averaged across night for each electrode. Diffusion images where denoised for rician noise8 and motion correted using FSL eddy.9 The fiber orientation distribution functions (spherical harmonic order 8) were computed using constrained spherical deconvolution10 and streamlines were obtained using anatomically constrained particle filter tractography.11 The T1 image was registered to the motion corrected b0 image and segmented using FreeSurfer.12 Streamlines were clustered based on their starting and ending regions and outliers were removed using QuickBundles.13,14 In addition to the thalamus, three cortical regions of interest were considered because of their proximity to the electrodes: the superior frontal gyrus, the middle frontal gyrus and the anterior cingulate cortex. To reduce the size of the regions of interest, the superior frontal gyrus was further segmented into 3 sub-regions as illustrated in Figure 1. Linear regression analyses were performed between the median length of the streamlines connecting the thalamus to each cortical region and the sleep spindle amplitudes and frequencies.Results
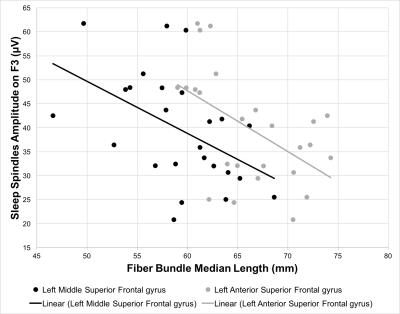
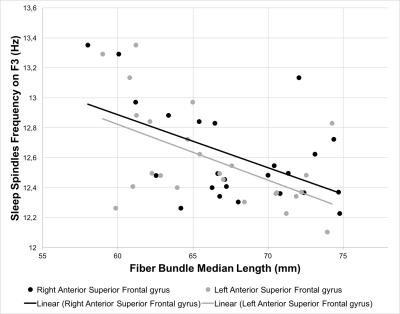
The length of the streamlines connecting the thalamus and the middle part of the left superior frontal gyrus significantly predicted sleep spindle amplitudes on F3, F4, C3, and C4 (β ranging from -0.465 to -0.503, p < 0.05) whereas the streamlines connecting to the anterior part of the left superior frontal gyrus predicted the amplitudes only on frontal derivations (F3: β = -0.525, p < 0.01; F4: β = -0.479, p < 0.05). Similarly, bilateral streamlines connecting the thalamus to the anterior part of the superior frontal gyrus significantly predicted sleep spindles frequency on F3, F4, C3, and C4 (left part: β ranging from -0.389 to -0.540, p < 0.05; right part: β ranging from -0.412 to -0.559, p < 0.05). No significant associations were detected between the sleep spindles amplitude or frequency and the length of the streamlines connecting the thalamus to the middle frontal gyrus and the anterior cingulate cortex.Discussion and conclusion
In this preliminary work, we investigated the association between the anatomical structure of the thalamo-cortical loop and features of sleep oscillations. Our results are in agreement with previous results which link sleep-related EEG events and white matter characteristics. However, our metric being streamline bundle specific, we highlight white matter fiber bundles connecting the thalamus to the frontal cortex which may be associated with sleep spindles. More specifically, we found that the length of the streamlines connecting the thalamus to the anterior portion of the superior frontal gyrus significantly predicted both sleep spindle amplitudes and frequencies. This promising approach may allow to further increase our understanding of sleep regulation mechanisms.Acknowledgements
No acknowledgement found.References
1. Schabus M, Gruber G, Parapatics S, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27(8):1479-1485.
2. Schabus M, Hodlmoser K, Gruber G, et al. Sleep spindle-related activity in the human EEG and its relation to general cognitive and learning abilities. Eur J Neurosci. 2006;23(7):1738-1746.
3. Ulrich D. Sleep spindles as facilitators of memory formation and learning. Neural Plast. 2016;2016:1796715.
4. Fogel SM and Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011;35(5):1154-1165.
5. Clawson BC, Durkin J and Aton SJ. Form and function of sleep spindles across the lifespan. Neural Plast. 2016;2016:6936381.
6. Piantoni G, Poil SS, Linkenkaer-Hansen K, et al. Individual differences in white matter diffusion affect sleep oscillations. J Neurosci. 2013;33(1):227-233.
7. Martin N, Lafortune M, Godbout J, et al. Topography of age-related changes in sleep spindles. Neurobiol Aging. 2013;34(2):468-476.
8. Descoteaux M, Wiest-Daesslé N, Prima S, et al. Impact of rician adapted non-local means filtering on HARDI. Paper presented at Medical Image Computing and Computer-Assisted Intervention (MICCAI). 2008:122-130.
9. Andersson JL and Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063-1078.
10. Tournier JD, Calamante F and Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: Non-negativity constrained super-resolved spherical deconvolution. Neuroimage. 2007;35(4):1459-1472.
11. Girard G, Whittingstall K, Deriche R, et al. Towards quantitative connectivity analysis: reducing tractography biases. Neuroimage. 2014;98:266-278.
12. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179-194.
13. Garyfallidis E, Brett M, Correia MM, et al. QuickBundles, a method for tractography simplification. Front Neurosci. 2012;6:175.
14. Cote M-A, Garufallidis E, Larochelle H, et al. Cleaning up the mess: tractography outlier removal using hierarchical QuickBundles clustering. Proceeding of: International Society of Magnetic Resonance in Medicine (ISMRM). Toronto, Canada, 2015.
Figures