4699
High resolution volumetric 3D-SoS MPRAGE detection of carotid intraplaque hemorrhage1Radiology, University of Utah, Salt Lake City, UT, United States, 2Neurology, University of Utah, Salt Lake City, UT, United States, 3Surgery, Salt Lake City VA Medical Center
Synopsis
To provide a better method of detection and quantification of carotid intraplaque hemorrhage (IPH), we developed a motion-robust 3D radial stack of stars (SoS) MPRAGE sequence. 3D SoS MPRAGE outperformed 3D TOF in the detection and quantification of carotid IPH when compared with histology. The sequence was robust to motion and flow related artifacts. This sequence, when combined with high-SNR neck shape specific coils, can help identify stroke etiology, determine future stroke risk, and evaluate treatment response by quantifying IPH volume in future clinical trials aimed at decreasing carotid IPH.
Clinical Question
Can we provide a better method to detect and quantify carotid intraplaque hemorrhage (IPH) in patients with carotid disease?Impact
Cardiovascular disease and stroke are among the leading causes of morbidity and mortality in the United States, with total yearly costs exceeding $300 billion.(1) Atherosclerosis is the cause of approximately 10-25% of the ischemic strokes in the United States every year.(1) Determination of stroke risk and management is based heavily on percent diameter stenosis detected by imaging. Our group and others have found that IPH identifies vulnerable carotid plaque better than stenosis.(2) Patients with carotid IPH have a high risk of stroke independent of stenosis, and are refractory to standard medical therapy, with a high hazard ratio of repeat stroke and annual risk ranging from 15% to 45%.(3-5) Research by our group and others has shown that carotid IPH detection yields immediate clinical impact by identifying stroke etiology, determining future stroke risk with the potential to evaluate treatment response in future clinical trials.
These studies place carotid IPH detection at the forefront of clinical decision-making in stroke patients. Many T1-weighted sequences have been used to detect IPH, including conventional T1w, 3D TOF, and MPRAGE and its variants. Of these, MPRAGE has consistently outperformed the others with a high sensitivity, specificity and accuracy >90% using a threshold signal intensity over adjacent muscle of 2-fold. Still, some clinicians continue to use other methods to identify stroke risk, either with lumen imaging alone or with combined lumen/IPH detection sequences including 3D TOF. Whichever technique used, carotid MRI sequences can be severely degraded due to patient motion, incomplete separation of IPH and flow-related enhancement, and difficulties determining positive MPRAGE signal above the adjacent muscle signal threshold of 2-fold. To fully realize the impact of carotid MRI, IPH detection must be accurate and robust to artifacts.
Approach
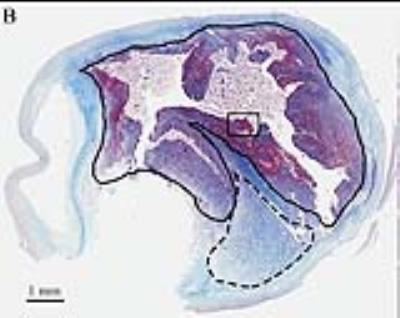
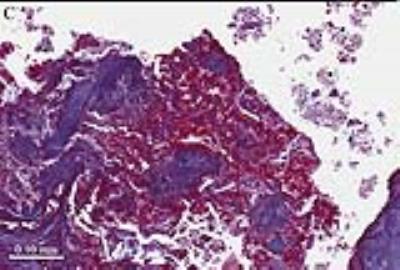
Our approach uses a motion-robust 3D radial stack of stars (SoS) MPRAGE sequence to detect and quantify carotid IPH in patients undergoing future carotid surgery. A total of 60 patients underwent informed consent prior to this study. We compared 3D SoS MPRAGE with 3D TOF as the current clinical standard, and results were further compared to histology to confirm the presence of IPH (Figures 1-3).
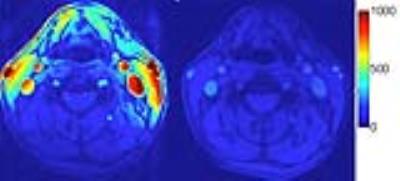
3D TOF and modified MPRAGE sequences used our innovative combined head/neck MRI as follows: First, the use of a combined modular head/neck radiofrequency coil permits high SNR imaging from the aortic arch to distal intracranial vessels in a single time-efficient scan. Using patient-fitting RF coils results in consistently higher SNR for evaluating arterial disease of the neck compared to standard commercial head and neck coils (Figure 4). To overcome image degradation by swallowing, cardiac, and respiratory motion, we employ novel multi-spectral MRI pulse sequences with IPH detected using 3D SoS MPRAGE with flow suppression, motion detection, and artifact correction and compared with conventional 3D TOF using the same head/neck coils (see 10 examples in preliminary data). The motion-robust high-resolution 3D SoS MPRAGE sequence consistently obtained superior images with reduced motion artifact, preserved SNR and accurate detection of IPH allowing quantification of IPH volume.
Gains and Losses
First, the 3D SoS MPRAGE protocol can be acquired during the same lumen imaging protocol, is performed without contrast and adds no additional risk. Most importantly, the 3D SoS MPRAGE sequence easily outperforms 3D TOF in IPH detection and quantification in all patients. 3D SoS MPRAGE is more robust to motion and can easily separate IPH from blood flow signal compared to 3D TOF. By detecting IPH, this adds significantly more information on carotid stroke sources and stroke risk independent of stenosis. Further, by quantifying IPH volume, 3D SoS MPRAGE adds the ability to monitor treatment response and investigate novel therapeutic agents through clinical trials. The addition of 3D SoS-MPRAGE comes at a cost of imaging time of 3:16 minutes, but our protocol mitigates this loss by simultaneously imaging the head/neck with high-SNR neck shape specific coils.Preliminary Data
Image quality for IPH detection and quantification is increased between 3D TOF and 3D SoS MPRAGE sequences using neck shape specific coils. The 10 representative images demonstrate the 3D TOF combined lumen and IPH imaging compared to the new 3D SoS MPRAGE where high signal indicates carotid IPH, and can be easily detected and quantified in patients with carotid disease. By diagnosing carotid IPH, the 3D SoS MPRAGE sequence adds significant clinical value, by determining unstable carotid plaque. This identifies potential stroke sources and provides a means of following treatment response by volumetric quantification in future trials.Acknowledgements
Supported by R01 HL127582, RSNA Research Scholar Grant RSCH1414, Siemens Medical Solutions, The Ben B. and Iris M. Margolis Foundation, and the Clinical Merit Review Grant from the Veterans Administration health Care System.References
1. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart Disease and Stroke Statistics--2013 Update: A Report From the American Heart Association. Circulation 2013;127(1):e6-e245.
2. McNally JS, McLaughlin MS, Hinckley PJ, Treiman SM, Stoddard GJ, Parker DL, Treiman GS. Intraluminal thrombus, intraplaque hemorrhage, plaque thickness, and current smoking optimally predict carotid stroke. Stroke; a journal of cerebral circulation 2015;46(1):84-90.
3. Hosseini AA, Kandiyil N, Macsweeney ST, Altaf N, Auer DP. Carotid plaque hemorrhage on magnetic resonance imaging strongly predicts recurrent ischemia and stroke. Annals of neurology 2013;73(6):774-784.
4. Saam T, Hetterich H, Hoffmann V, Yuan C, Dichgans M, Poppert H, Koeppel T, Hoffmann U, Reiser MF, Bamberg F. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. Journal of the American College of Cardiology 2013;62(12):1081-1091.
5. Gupta A, Baradaran H, Schweitzer AD, Kamel H, Pandya A, Delgado D, Dunning A, Mushlin AI, Sanelli PC. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke; a journal of cerebral circulation 2013;44(11):3071-3077.
Figures