4697
Prenatal exposure to phthalate esters and later brain structure change revealed by generalized q-sampling MRI1Department of Medical Imaging and Radiological Sciences, Chung Shan Medical University, Taichung, Taiwan, 2Department of Medical Imaging, Chung Shan Medical University Hospital, Taichung, Taiwan, 3School of Medicine and Department of Pediatrics, Chung Shan Medical University and Hospital, Taichung, Taiwan, 4Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan, 5National Institute of Environmental Health Sciences, National Health Research Institutes, Miaoli, Taiwan
Synopsis
Phthalate esters are a group of chemicals that are widely used everywhere. There is an emerging public health issue that the prevalent use of phthalates may affect children’s brain development. Therefore, we tried to use generalized q-sampling imaging (GQI) to identify the neurological structure changes of white matter of children’s brain induced by prenatal phthalate exposure. The altered GQI indices in the corpus callosum, corona radiata, superior longitudinal fasciculus (SLF), internal capsule, and superior frontal gyrus were found in the children’s brain who were prenatal exposure to phthalate esters.
Introduction
Phthalate esters are a group of chemicals that are widely used in industrial applications and consumer products. For instance, di-(2-ethylhexyl) phthalate (DEHP) is common in polyvinyl chloride (PVC) products, including food packaging, clothing, building materials and children’s toys. However, these chemicals can enter the human body by inhalation and ingestion. Therefore, the prevalent use of phthalates has become a public health issue, which may affect brain development and children’s behavior1. Previous studies mentioned that the influence of rat brain exposure to prenatal phthalate2, however, few studies focused on the human brain. Therefore, we used generalized q-sampling imaging (GQI), a useful and promising technique in investigating the neurological underpinnings of the disorder, to identify the neurological structure changes of the white matter of children’s brain induced by prenatal phthalate exposure.Materials and Methods
In the study, we examined the association between phthalate esters in the maternal urine collected during the third trimester of pregnancy and the children's brain MRI. All of 48 participants, including 27 males (age 13-16 years) and 21 females (age 13-14 years) were acquired. All participants performed a brain MRI examination on 3T imaging system (Skyra, Siemens, Germany). The scanning parameters: TR/TE = 4800/97 ms, signal average = 1, slices=35, voxel size = 2 x 2 x 4 mm3, 192 diffusion direction with b-values of 1000, 1500, 2000 s/mm2 and 10 null imaging, and scan time was about 17 minutes. The raw diffusion data for each participant were processed with eddy current correction using FSL (FMRIB Software Library). Then, the corrected diffusion images were spatially normalized to the Montreal Neurological Institute (MNI) T2W template using parameters determined from the normalization of the diffusion null image to the T2W template using Statistical Parametric Mapping 8 (SPM8, Wellcome Trust Centre for Neuroimaging, UK). GQI reconstruction was performed using DSI Studio (National Taiwan University, Taipei, Taiwan), and the generalized fractional anisotropy (GFA), normalized quantitative anisotropy (NQA) and isotropic of the orientation distribution function (ISO) mapping were calculated. For the statistical analysis, multiple regression was used to obtain the correlation between the GQI indices and the mother’s creatinine-corrected urine concentrations of prenatal phthalate metabolites, including DEHP, mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), mono-benzyl phthalate (MBzP) and mono-butyl phthalate (MBP), for the 48 participants. In addition, gender was used as the covariates.Results
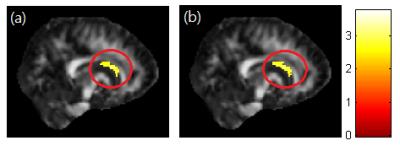
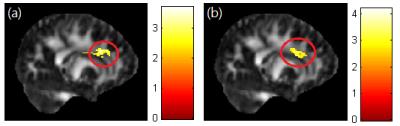
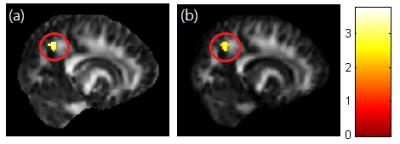
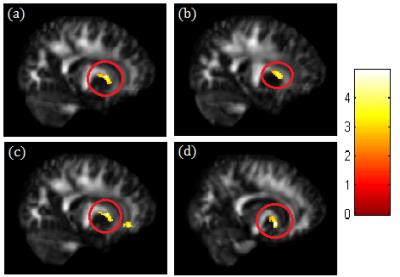
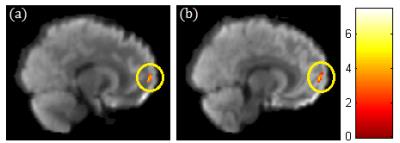
A significant negative correlation between DEHP/MEOHP and GFA in the corpus callosum (p<0.01) were observed (Fig. 1). A significant negative correlation between DEHP/MBzP and GFA in the corona radiata (p<0.01) were also found (Fig. 2). A significant negative correlation between MBP and GFA/NQA in the superior longitudinal fasciculus (SLF) (p<0.01) were observed (Fig. 3). Moreover, a significant negative correlation between DEHP/MEHP/MEOHP/MBzP and NQA in the internal capsule (p<0.01) were found (Fig. 4). In addition, we observed a significant positive correlation between DEHP/MEOHP and ISO in the superior frontal gyrus (p<0.02) (Fig. 5).Discussion
Lower GFA in corpus callosum when exposed to DEHP and MEOHP may indicate the worse inter-hemispheric functional integration of cognitive, learning, executive, attention and emotional processes3. Recent evidence suggests that reduced volume of the corpus callosum are related to depression and suicide attempts in mood disorders4. Lower GFA in corona radiata when exposed to DEHP and MBzP may associate with depression, irritability, and aggressive behavior5. Lower GFA and NQA in SLF when exposed to MBP may associate with cognitive function and mood regulation6. The previous study in late-life depression revealed decreased FA in SLF with later age of onset, which might be explained by a vascular hypothesis of depression7. Lower NQA in the internal capsule when exposed to DEHP, MEHP, MEOHP, and MBzP may associate with depression, phobia, and compulsive disorder8. Higher ISO in superior frontal when exposed to DEHP and MEOHP may subserve the formation and emotional processing of long-term memories, as well as modulates emotional responses for social appropriateness9. The previous study also mentioned the impact of prenatal phthalate exposure on children’s behavior, including depression, attention, aggression and externalizing behaviors1. One cross-sectional study revealed that exposure to DEHP may associate with attention deficit/hyperactivity disorder (ADHD) symptoms10, and altered WM integrity was most evident in the corpus callosum, corona radiata, SLF and internal capsule11.Conclusion
Our results showed decreased GFA or NQA in the corpus callosum, corona radiata, SLF, internal capsule and increased ISO in the superior frontal gyrus of the children’s brain who were prenatal exposure to phthalate esters. These findings suggest that prenatal phthalate exposure might play an important role in the development of brain later in the children’s lives. Phthalate esters are new prospects and challenges for near future research into the neurological structure of the human brain.Acknowledgements
This study was supported in part by the nationwide Taiwan Maternal and Infant Cohort Study.References
1. Lien YJ, Ku HY, Su PH, et al. Prenatal Exposure to Phthalate Esters and Behavioral Syndromes in Children at 8 Years of Age: Taiwan Maternal and Infant Cohort Study. Environmental Health Perspectives. 2015; 123(1): 95-100.
2. Li XJ, Jiang L, Chen L, et al. Neurotoxicity of dibutyl phthalate in brain development following perinatal exposure: a study in rats. Environ Toxicol Pharmacol. 2013; 36(2): 392–402.
3. Ozalay O, Calli C, Kitis O, et al. The relationship between the anterior corpus callosum size and prefrontal cortex volume in drug-free depressed patients. Journal of Affect Disorder. 2013; 146(2): 281–285.
4. Taylor WD, Boyd B, McQuaid DR, et al. Widespread white matter but focal gray matter alterations in depressed individuals with thoughts of death. Neuro-Psychopharmacology and Biological Psychiatry. 2015; 62: 22–28.
5. Shimoda K, Kimora M. Two cases of emotional disorder after middle cerebral artery infarction showing distinct responses to antidepressant treatment. 2014; 10: 965–970.
6. Makris N, Kennedy DN, McInerney S, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cerebral Cortex. 2005; 15: 854–869.
7. Sexton CE, Masurier ML, Allan CL, et al. Magnetic resonance imaging in late-life depression: vascular and glucocorticoid cascade hypotheses. The British Journal of Psychiatry. 2012; 201(1): 46-51.
8. Doshi PK. Surgical treatment of obsessive compulsive disorders: Current status. Indian Journal of Psychiatry. 2009; 51(3): 216–221.
9. Stuss DT, Gow CA, Hetherington CR. “No longer Gage”: frontal lobe dysfunction and emotional changes. Journal of Consulting and Clinical Psychology. 1992; 60(3): 349-359.
10. Kim BN, Cho SC, Kim Y, et al. Phthalates exposure and attention-deficit/ hyperactivity disorder in school-age children. Biological Psychiatry. 2009; 66(10): 958–963.
11. Ewijk HV, Heslenfeld DJ, Zwiers MP, et al. Diffusion tensor imaging in attention deficit/hyperactivity disorder: A systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews. 2012; 36: 1093–1106.
Figures