4695
Relationship between nicotine dependence and the structural changes in the brain of young and middle-aged male smokers: a voxel-based morphometry study1Radiology, Beijing Chao-Yang Hospital, Beijing, People's Republic of China, 2School of Biomedical Engineering, Capital Medical University, Beijing, People's Republic of China, 3MR Scientific Marketing, Siemens Healthcare Ltd. Diagnostic Imaging
Synopsis
This study aimed to research the relationship between the volume changes of brain and smoking extent/nicotine dependence. A voxel-based morphometry study between smokers grouped by smoking amount and dependence level was conducted. This study demonstrated the volume of many brain areas decreased with the increase in smoking extent and an opposite correlation between some brain areas and smoking dependence was observed. This study indicated that dependence had limited ability to predict smoking-related brain structure changes. A comprehensive assessment of the situation should be consulted, when clinicians make treatments options.
Purpose
Purpose: Many previous studies have demonstrated that smokers exhibit changes in the volume of some brain areas with the amount of smoking (pack-years), which was an effective indicator of these changes. However, there is a no solid consensus about the relationship between nicotine dependence level and smoking-related structural changes; the variations in sample and analysis methods were reported as the causative reason (1-6). Therefore, based on the definition used by many large epidemiological surveys, the purpose of this study was to examine the presence of the negative relationship between lifetime tobacco consumption and nicotine dependence and grey-matter and white-matter volume in young and middle-aged male smokers.
Methods
Methods: Data were collected on a MAGNETOM Trio Tim 3T MR scanner (Siemens, Erlangen, Germany) with an 8-channel head coil. In total, 53 long-term male smokers (mean age: 30.7±4.2 years, range: 23-40 years) underwent a whole-brain high-resolution T1WI-3D MPRAGE sequence scan using the following parameters: TR/TE=1900/2.52 ms; total slices=176; slice thickness=1 mm; matrix=250×250; flip angle=9°; in-plane voxel size=1×1×1 mm3; total acquisition time=5:40 min. The subjects were categorized as light (pack-years 2.5-19.5, N=26) and heavy smokers (pack-years 21-48, N=27) as well as those with respect to their nicotine dependence using the Fagerström Test for Nicotine Dependence (FTND): mild/moderate (FTND 1-7, N=23) and severe (FTND 8-10, N=30). The data were processed using SPM8, in which VBM was implemented using the DARTEL Tools set to the default parameters. The scan images were preprocessed for elements including bias correction, tissue classification, and data registration using linear (12-parameter affine) and non-linear (warping) transformations, featuring a unified model. Analyses were subsequently performed on the GM and WM segments. The brain volume was compared between individuals with different smoking duration and different nicotine dependence by setting pack-years and FTND as covariates (F-test/ANCOVA, AlphaSim-corrected to p<0.05, cluster size>26).Results
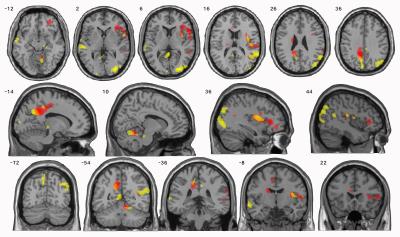
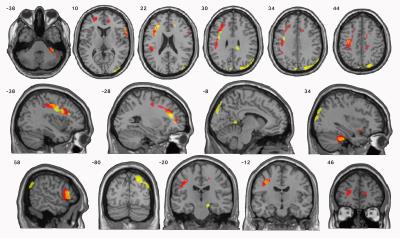
Results: The volume of many brain areas decreased with the increase in smoking extent. This volume change was more prominent in some specific areas of grey matter of precuneus, inferior and middle frontal gyrus and insula, and areas of white matter of precentral gyrus, cerebellum anterior lobe and inferior frontal gyrus. However, an opposite correlation was observed between brain volume changes and the severity of nicotine dependence, especially in specific areas of grey matter of precuneus, superior temporal gyrus and middle occipital gyrus, and areas of white matter of precuneus, middle occipital gyrus and inferior frontal gyrus (Table1, 2; Figure1, 2).Discussion
Discussion: As per our knowledge, this is the first study to analyze the relationship between brain structure changes and smoking extend/nicotine dependence in the same group of smokers. In this study, we targeted on the main smoking population in China, the young and middle-aged male smokers. Interesting, we observed that the extent of smoking and dependence level have opposite correlation with brain volume. Moreover, there were some intersecting subjects in this study. Some heavy smokers had only mild/moderate dependence while some light smokes have severe dependence. This may be because extent of smoking directly reflects the intake of harmful substances in tobacco, on the other hand, nicotine dependence is a phenomenon that does not automatically increase with the number of years smoking and the extend of smoking (7). In addition, genetics and environmental factors play a significant role in nicotine dependence (8-10). FTND is one of the most commonly used measures of nicotine dependence, but considerable research has shown that the FTND has poor internal consistency and poor reliability, and a limited ability to predict smoking-related outcomes (11-14). The Chinese guidelines for the cessation treatment of smoking suggest that patients with mild/moderate dependence can give up smoking using willpower alone (15). Although in China most smokers only have mild nicotine dependence, smokers with mild/moderate dependence may already have severe brain damage (16,17). Also, Chinese culture and social communicative habits make it hard to quit smoking for smokers. Therefore, physicians should also provide more active smoking cessation interventions to smokers with mild dependence to help them quit as soon as possible. When combined with the FTND, both pack-years and MRI findings can help physicians to more accurately determine the health status of smokers.Conclusions
Conclusions: A higher level of cigarette smoking appears to be associated with brain-volume atrophy, but no such association was observed with the nicotine dependence.Acknowledgements
We thank the World Health Organization Collaborating Centre for Tobacco or Health and the Beijing Institute of Respiratory Medicine for their assistance with the recruitment of smokers and data collection.References
1. Karama S, Ducharme S, Corley J, et al. Cigarette smoking and thinning of the brain’s cortex. Mol Psychiatry. 2015;20(6): 778–785.
2. Smith CD, Chebrolu H,Wekstein DR, et al. Age and gender effects on human brain anatomy: a voxel-based morphometric study in healthy elderly. Neurobiol Aging. 2007;28(7): 1075–1087.
3. Power MC, Deal JA, Sharrett AR, et al. Smoking and white matter hyperintensity progression: the ARIC-MRI Study. Neurology. 2015;84(8): 841–848.
4. Pennington DL, Durazzo TC, Schmidt TP, et al. Alcohol use disorder with and without stimulant use: brain morphometry and its associations with cigarette smoking, cognition, and inhibitory control. PloS one. 2015;10(3): e0122505.
5. Li Y, Yuan K, Cai C, et al. Reduced frontal cortical thickness and increased caudate volume within fronto-striatal circuits in young adult smokers. Drug Alcohol Depend. 2015;151: 211–219.
6. Prom-Wormley E, Maes HH, Schmitt JE, et al. Genetic and environmental contributions to the relationships between brain structure and average lifetime cigarette use. Behav Genet. 2015;45(2): 157–170.
7. John U, Meyer C, Hapke U, et al. The Fagerstrom test for nicotine dependence in two adult population samples-potential influence of lifetime amount of tobacco smoked on the degree of dependence. Drug Alcohol Depend. 2003;71(1):1-6.doi:10.1016/S0376-8716(03)00038-3.
8. Li MD. The genetics of smoking related behavior: a brief review. Am J Med Sci. 2003;326(4):168-173. doi:10.1097/00000441- 200310000- 00003.
9. Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine Tob Res.1999;1 Suppl 2: p. S51-7. doi:10.1080/ 1462229905 0011811.
10. Li MD, Cheng R, Ma JZ, et al. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction.2003;98(1):23-31. doi: 10.1046/j. 1360-0443. 2003.00295.x.
11. Etter JF, Duc TV, Perneger TV. Validity of the Fagerstrom test for nicotine dependence and of the Heaviness of Smoking Index among relatively light smokers. Addiction. 1999;94(2):269-281. doi: 10.1046/j.1360-0443.1999.94226910.x.
12. Hughes JR, Oliveto AH, Riggs R, et al. Concordance of different measures of nicotine dependence: two pilot studies. Addict Behav. 2004;29(8):1527-1539. doi:10.1016/j.addbeh.2004.02.031.
13. Sledjeski EM, Dierker LC, Costello D, et al. Predictive validity of four nicotine dependence measures in a college sample. Drug Alcohol Depend. 2007;87(1):10-19.doi:10.1016/j.drugalcdep.2006.07.005.
14. Pomerleau CS, Carton SM, Lutzke ML, et al. Reliability of the Fagerstrom Tolerance Questionnaire and the Fagerstrom Test for Nicotine Dependence. Addict Behav. 1994;19(1):33-39. doi:10.1016/0306-4603(94)90049-3.
15. Ministry of health PRC, editor. China Report on the Health Hazards of smoking. Beijing, China: People's medical publishing house; 2012. 246 p.
16. Chen GB, Zou XN, Chen YL, et al. Investigation of Nicotine Dependence and Its Influencing Factors of Smokers in China. Medicine and Society. 2013;26(12):1-4.
17. Yang T, Shiffman S, Rockett IR, et al. Nicotine dependence among Chinese city dwellers: a population-based cross-sectional study. Nicotine Tob Res.2011;13(7):556-564. doi: 10.1093/ntr/ntr040.
Figures