4678
Voxel-based analysis of grey and white matter degeneration above the level of injury in cervical spinal cord injury1University Hospital Balgrist, University of Zurich, Zurich, Switzerland, 2Department of Neurophysics, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 3Wellcome Trust Centre for Neuroimaging, UCL Institute of Neurology, University College London, London, United Kingdom, 4of Systems Neuroscience, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, 5Department of Brain Repair and Rehabilitation, UCL Institute of Neurology, University College London, London, United Kingdom
Synopsis
We studied the extent of cervical grey and white matter neurodegeneration above the level of injury after traumatic spinal cord injury (SCI) using high-resolution structural and diffusion MRI data. We found marked atrophy of both white and grey matter alongside diffusivity changes associated with axonal degeneration within the major spinal tracts. The extent of structural decline related to clinical impairment. These tract specific changes and clinicopathological relationships shed light into underlying neurodegenerative disease mechanisms, and therefore these measureable changes hold potential to serve as neuroimaging biomarkers of cord pathology.
Target Audience
Researchers and clinicians interested in applications of MRI of the spinal cord, particularly after spinal cord injury.Purpose
Spinal cord injury (SCI) is a devastating life event, which in most cases leads to sensorimotor impairment. Alongside the functional impairments, the spinal cord and brain undergo extensive neurodegeneration above the level of injury1. However, it is unclear to what extent pathological processes occur within the grey and white matter of the cervical spinal cord. We used high-resolution spinal cord neuroimaging2 to investigate tract-specific neurodegeneration above the level of injury in cervical grey and white matter, and its relation to clinical impairment in chronic SCI patients.Methods
Seventeen tetraplegic SCI patients (mean/SD: 48.7years ± 14.1) and 22 healthy controls (41.1years ± 11.4) were recruited. MRI was assessed in all subjects using a 3T SkyraFit scanner (Siemens, Germany). At the cervical cord (C2/C3) above the level of injury, a 3D high-resolution optimized T2*-weighted multi-echo sequence (MEDIC)3 was acquired (resolution: 0.25×0.25×2.50mm3; FOV: 162×192mm2; matrix size: 648×768; TR/TE: 44/19ms; flip angle: α=11°; BW: 260 Hz/pixel; acquisition time: 2min8s/volume, 5 volumes total), as well as a cardiac-gated4 single shot spin-echo EPI sequence (6 directions: b=0; 30 directions: b=500s/mm2; slice thickness: 5mm; 5/8 Partial-Fourier Imaging in phase-encoding direction; acquisition matrix: 176×40; FOV: 133×30mm2; in-plane resolutions: 0.8×0.8mm2; TE/TR= 73/350ms; acquisition time: 6.2min).
The MEDIC sequence was used to segment cross-sectional spinal cord area
(SCA), grey matter area (GMA) and white matter area (WMA) using a surface model
in combination with fuzzy connector segmentation as implemented in JIM 6.0 (http://www.xinapse.com).
DTI was interpolated to a higher in-plane resolution of 0.4×0.4mm2
and subsequently motion and eddy current corrected5. Afterwards, we
used robust fitting as implemented in the ACID toolbox and calculated fractional
anisotropy, as well as radial, axial and mean diffusivity. These DTI index maps
were then spatially normalized to a self-constructed template located in the
spinal MNI space.
We used STATA (StataCorp LP, USA) to assess group differences with a Mann-Whitney-U-test (p<0.05). Linear regression models were used to determine associations between morphometry and clinical outcome, adjusted for age. SPM12 was used to perform voxel-based analysis of the different DTI indices, corrected for age. All statistical parametric maps were initially thresholded with a cluster defining threshold of p<0.01 (uncorrected). Clusters surpassing a cluster threshold of p=0.05, corrected for family-wise error are reported.
Results
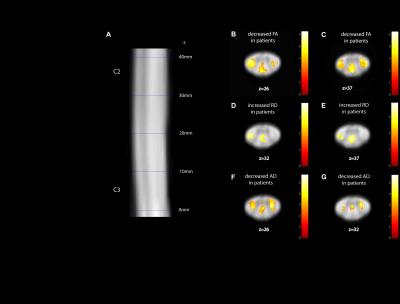
Compared to controls, patients showed a reduced SCA of 20.2% (p=0.006), reduced GMA of 30.0% (p<0.0001) and reduced WMA of 16.9% (p=0.0266) (Fig 1). Voxel-based analysis of the cervical cord revealed a decreased FA and AD in the left corticospinal tract (FA: p=0.003; z score: 4.42; cluster extent: 154; AD: p=0.014; z score: 3.72; cluster extent: 58), the right corticospinal tract (FA: p=0.025; z score: 4.34; cluster extent: 85; AD: p=0.002; z score: 4.70; cluster extent: 94) and the dorsal columns (FA: p=0.004; z score: 3.80; cluster extent: 145; AD: p=0.020; z score: 3.69; cluster extent: 52) in patients compared to controls (Fig 2). RD was increased in the left corticospinal tract (p=0.023; z score: 3.22; cluster extent: 69) and dorsal columns (p=0.022; z score: 3.47; cluster extent: 70) in patients compared to controls. MD was not significantly different.
A smaller SCA was associated with worse lower extremity motor (p=0.035, R2=0.265), light-touch (p=0.035, R2=0.264), and pin-prick scores (p=0.029, R2=0.280). A smaller GMA area was associated with worse lower and upper extremity motor (lems: p=0.036, R2=0.279; uems: p=0.030, R2=0.293), pin-prick (p=0.001, R2=0.536) and SCIM scores (p=0.035, R2=0.264). Lower FA (p<0.001; z score: 4.07; cluster extent: 394) and higher RD values in the dorsal columns (p<0.001; z score: 3.83; cluster extent: 377) were associated with worse SCIM scores. Lower FA (p=0.013; z score: 4.07; cluster extent: 114) and higher RD in the dorsal columns (p=0.017; z score: 3.45; cluster extent: 103) were associated with worse light-touch scores. Lower FA in the dorsal column was associated with worse GRASSP sensory score (p=0.022; z score: 3.78; cluster extent: 46).
Discussion/ Conclusion
This study used spinal cord neuroimaging to reveal spatially localized cord pathology. Atrophy of both white and grey matter was evident above the level of injury. Within areas of white matter atrophy, microstructural diffusivity changes associated with axonal degeneration and demyelination were observed. Crucially, the magnitude of tract-specific retrograde and anterograde neurodegeneration within the main motor and sensory tracts was related to clinical impairment of motor (muscle strength) and sensory (light-touch) function. Thus, these neuroimaging biomarkers could complement conventional clinical scores and might help to monitor the course of rehabilitation and reveal subtle treatment effects in clinical trials.Acknowledgements
No acknowledgement found.References
1. Freund P et al. MRI investigation of the sensorimotor cortex and the corticospinal tract after acute spinal cord injury: a prospective longitudinal study. Lancet Neurol. 2013:12:873–81.
2. Wheeler-Kingshott CA et al. The current state-of-the-art of spinal cord imaging: applications. Neuroimage. 2014:84:1082–1093.
3. Schmid MR et al. Imaging of patellar cartilage with a 2D multiple-echo data image combination sequence. Am J Roentgenol. 2005:184:1744–1748.
4. Morelli J. N. et al. Evaluation of a modified Stejskal-Tanner diffusion encoding scheme, permitting a marked reduction in TE, in diffusion-weighted imaging of stroke patients at 3 T. Invest. Radiol. 2010:45:29–35.
5. Mohammadi S. et al. High-resolution diffusion kurtosis imaging at 3T enabled by advanced post-processing. Front. Neurosci. 2015:8:1–14.