4676
Free Water Estimation Improves in vivo Diffusion MRI Remote from the Lesion Site in Rat Spinal Cord Injury1Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, United States, 2Medical College of Wisconsin, Milwaukee, WI, United States, 3Biomedical Engineering, Marquette University, Milwaukee, WI, United States
Synopsis
Diffusion tensor imaging (DTI) of the spinal cord remote from the site of injury has been shown to be a surrogate marker of spinal cord injury (SCI) severity. In this work, detection of injury severity with in vivo diffusion MRI of the rat cervical cord following a thoracic SCI was improved with advanced diffusion MRI signal modelling compared to DTI.
Purpose
With its ability to measure and detect microscopic tissue changes noninvasively, diffusion weighted imaging (DWI) has shown promise as marker of spinal cord injury (SCI). However, imaging at the site of injury is plagued by challenges from susceptibility artifacts including hemorrhage and surgically-instrumented hardware. Recently, imaging remote from the site of injury was shown to be a surrogate marker of injury1. In this work, DWI of the cervical cord was performed in vivo at multiple points after a thoracic contusion SCI in a rat model using diffusion tensor imaging and free water estimation (FWE).Methods
MRI was performed on a Bruker 9.4 T Biospec using a 3.8 cm inner diameter Tx/Rx Litz volume coil (Doty Scientific) at 2, 30, and 90 days after a surgical contusion injury induced through weight drop2. Diffusion weighted images were acquired with a 4-shot EPI acquisition (TE=28 ms; TR=>1500 ms, varied by respiratory rate) with 25.6x25.6 mm2 field of view, matrix of 128x128, spatial resolution of 0.20x0.20 mm2 and 14 axial slices with a thickness of 1.0 mm (0.75 mm gap) spanning vertebral segments C1 to C7. A frequency-selective saturation pulse along with slice gradient reversal were used for fat saturation. Diffusion weighting employed Stejskal-Tanner gradients (δ/Δ=8.25/12.5 ms) along 30 directions at b-values of 250, 500, 1000, and 2000 s/mm2 and 15 non-diffusion-weighted images.
Preprocessing included EPI phase correction (custom Matlab) and motion and eddy current correction (FSL eddy). Diffusion parameter maps were calculated according to the diffusion tensor (DTI)3 and free water estimation (FWE)4 models for axial (AD), radial (RD), and mean diffusivity (MD), and fractional anisotropy (FA). Spatial alignment to a study-specific template was performed using iterative slice-by-slice spatial registration (ANTS and Spinal Cord Toolbox5), with regions of interest in white (WM) and gray matter (GM) manually traced on the mean maps in template space.
Mean ROI values were analyzed for a main effect of injury, time, or interaction using mixed-effect ANOVA (Stata). Linear regression analysis was performed to relate injury biomechanics (compression of the cord in mm) and functional scores (Basso, Beattie, and Breshnahan6; BBB) to the DWI metrics. All statistical tests used a significance level of p<0.05 and were corrected for multiple comparisons by controlling for the false discovery rate. For tests meeting significance, a subsequent voxelwise regression analysis was performed to reveal the spatial patterns of the functional relationships (FSL randomize).
Results
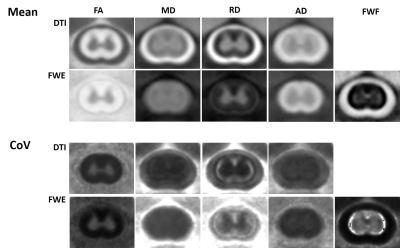
The FWE model improved the partial volume effects between the spinal cord WM and surrounding CSF(Fig. 1). The custom slice-by-slice spatial registration produced high-quality mean parameter maps, with inter-animal coefficient of variations of approximately 20% or less. Across all sham injury animals (n=7 x 3 timepoints), the free water fraction (FWF) was represented only a small portion of the white (4.9%) and gray (5.7%) signal. Compared to DTI, the FWE algorithm decreased the inter-animal coefficient of variation of AD (-7.6%) and MD (-9.7%), had only a minor effect on FA (+1.0%), but substantially increased the variation for RD (+67.9%), with similar trends apparent for GM.
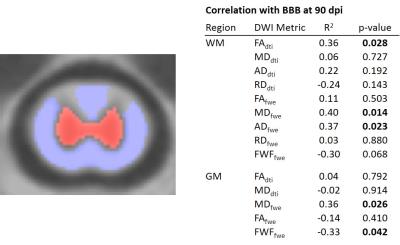
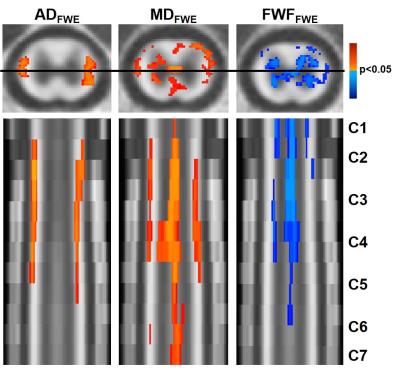
In the WM, MDFWE (F=4.83; p=0.007) and ADFWE (F=3.06; p=0.041) demonstrated a significant main effect of injury at 90 days post injury, whereas in the GM, MDFWE (F=4.54; p=0.009) was significant at 90 days post injury. None of the DTI metrics had a significant effect of severity. In the regression analysis relating WM values with final BBB score(Fig. 2), FADTI(p=0.028), MDFWE(p=0.014), and ADFWE(p=0.023) were significant, whereas in the GM MDFWE(p=0.026) and FWF(p=0.042) were significant. A subsequent voxelwise regression analysis demonstrated distinct patterns of changes(Fig. 3). The relationship between ADFWE and functional outcome was clearly evident in the lateral columns of the white matter extending from C1 to C5, with ADFWE decreasing with greater functional impairment. On the other hand, FWF revealed the most consistent changes along the spinal cord central canal, with FWF increasing with greater functional impairment.
Discussion/Conclusions
The FWE-corrected AD improved sensitivity to injury severity in the chronic stages of injury (90 dpi), but not in the acute injury aftermath. A decrease in AD with greater injury is suggestive of axonal degeneration and inflammatory involvement in the chronic stages of injury. The increased FWF may be suggestive of local edema or syringomyelia formation caused by obstruction of flow of CSF at the injury site. Both features appear to reveal distinct mechanisms of traumatic SCI that affect remote regions of the cord. In addition to diagnostic targets, these features and their noninvasive detection with DWI, may serve as important therapeutic targets for both reparative and rehabilitative strategies.Acknowledgements
Project was partially funded through the Research and Education Initiative Fund, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin, the Craig H. Neilsen Foundation, and the Department of Veterans Affairs. NS is a member of the Medical Scientist Training Program at MCW, which is partially supported by a training grant from NIGMS T32-GM080202. This publication was partially supported by the National Center for Advancing Translational Science, National Institutes of Health, through grant numbers UL1TR001436 and 1TL1TR001437. Support from the Bryon Riesch Paralysis Foundation is gratefully acknowledged.References
1: Jirjis MB, et al. J Neurotrauma. 2013. 2: Skinner NP, et al. Magn Reson Med. 2016. 3: Basser PJ, et al. Biophys J. 1994. 4: Pasternak O, et al. Magn Reson Med. 2009. 5: De Leener B, Neuroimage. 2016. 6 Basso DM, et al. J Neurotrauma. 1995.
Figures