4672
Suppression of CSF-flow-artifacts for T1-w Spin-echo Spine Imaging1Neuroradiology, Karolinska University Hospital, Stockholm, Sweden, 2Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden
Synopsis
We propose a simple method to suppress cerebrospinal fluid pulsatile-flow-artifacts in axial 2D T1-weighted spin-echo images of the spine. Widening the spatial width of the excitation pulse is shown to remove artifacts that otherwise could obscure or mimic tumors in the spinal canal.
Purpose & Background:
Axial 2D T1-weighted spin-echo (SE) images of the spine acquired with slice gap or in two or more acquisitions suffer from cerebrospinal fluid (CSF) pulsatile-flow-artifacts, which arise when relaxed CSF flows into the slice being imaged [1]. The artifacts often obscure or mimic tumors in the spinal canal in clinical practice and are amplified in children where the CSF pulsates at a higher rate due to narrower ventricular space and higher heart rate.
To suppress any kind of in-flow, spatial saturation can be applied on both sides of the imaged slice in the slice encoding direction [2], but this preparation significantly increases scan time and SAR for sequences with short readouts.
We propose a simplistic solution to efficiently solve this problem, where we broaden the spatial width of the excitation pulse and let the refocusing pulse define the slice thickness. This keeps the surrounding tissues and fluids from relaxing between repetitions and thereby suppressing the signal from CSF flowing into the imaged slice. A similar approach has successfully been used to avoid CSF artifacts in FLAIR images [3].
Methods:
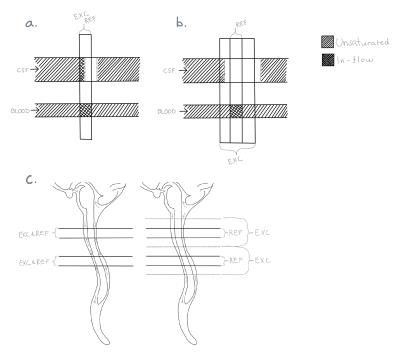
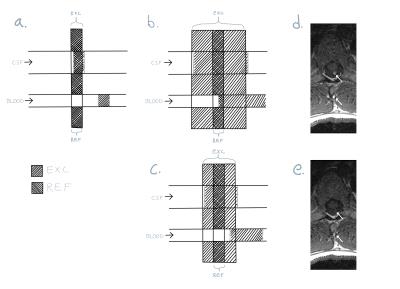
The excitation thickness was increased as much as possible without interfering with the neighboring slices, while keeping the refocused slice thickness as prescribed. Broader excitation thickness is possible as the scan is divided into more acquisitions (schematic drawing in Figure 1).
The slice thickness depends on the relationship between the radio frequency (RF) bandwidth and the slice selection gradient amplitude and can therefore be changed by modifying either of them. In this work, the gradient amplitude was kept constant and instead the RF excitation bandwidth was increased by shortening the RF pulse duration. Because the chemical shift only depends on the gradient amplitude, this ensures that the chemical shift in the slice direction is the same for the excitation and the refocusing pulses.
Modifications were made to an in-house developed fast spin-echo sequence and the scans were performed on a 1.5 T MRI system (DVMR450, GE Healthcare, Milwaukee, WI). Two volunteers, one 27 year old and one 12 year old, were examined with and without in-flow-suppression, after informed consent and IRB approval. Scan parameters for the T1-w FSE were as follows: FOV = 200×160 mm2, matrix = 320×206, slice thickness = 3 mm, Nslices = 45, exc./ref. FA = 90°/160°, TE/TR = 11/500 ms, ETL = 3, Nacq = 3 and rBW = ±30 kHz/FOV. An anterior spatial saturation pulse was applied to suppress motion artifacts from the lung and heart. All images were reconstructed with the vendor’s regular reconstruction pipeline
Results & Discussion:
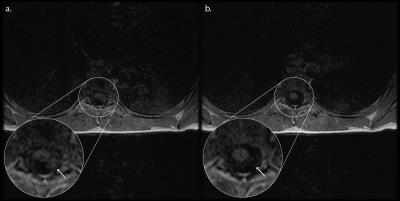
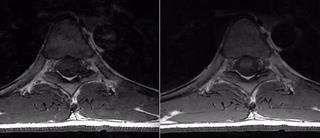
The performance of the proposed technique is shown in Figures. 2 and 3 for the 12 and 27 year old volunteers, respectively. A clear reduction in CSF pulsation artifacts is seen, since spins outside the refocusing slice have experienced only an excitation RF pulse and subsequent crusher and imaging gradients, but no refocusing RF pulse. Figure 3 illustrates that artifacts arising from the lungs and heart are better suppressed as well due to the thicker excitation thickness.
If the excitation pulse is becomes too wide, in-flow artifacts from blood will appear, because blood excited far from the refocused slice will not have enough time to flow through. This is illustrated in Figure 4. Unlike the case of CSF pulsation the blood signal will not be properly phase encoded.
A 3D sequence would achieve the same CSF suppression, but is more sensitive to body motion and may therefore be less ideal for pediatric imaging. The shortening of the excitation RF pulse adds a bit of flexibility when choosing the echo time. For example, shorter echo times can be achieved if used with only one echo (ETL = 1) and half-fourier in the readout direction. On the other hand the increased excitation bandwidth increases the peak power of the RF pulse and SAR, neither of which was a limiting factor in this work.
Acknowledgements
No acknowledgement found.References
1. Ståhlberg F, Ericsson A, Nordell B, Thomsen C, Henriksen O, Persson BR. MR imaging, flow and motion. Acta radiol. 1992;33: 179–200.
2. Edelman RR, Atkinson DJ, Silver MS, Loaiza FL, Warren WS. FRODO pulse sequences: a new means of eliminating motion, flow, and wraparound artifacts. Radiology. 1988;166: 231–236.
3. De Coene B, Hajnal JV, Gatehouse P, Longmore DB, White SJ, Oatridge A, et al. MR of the brain using fluid-attenuated inversion recovery (FLAIR) pulse sequences. AJNR Am J Neuroradiol. 1992;13: 1555–1564.
Figures