4671
Reduced FOV with Multi-shot IRIS Provides Improved Diffusion Tensor Imaging in the Cervical Spinal Cord1Biomedical Engineering, Vanderbilt University, Nashville, TN, United States, 2Vanderbilt University Institute of Imaging Science, Vanderbilt University Medical Center, Nashville, TN, United States, 3Philips Healthcare, Nashville, TN, United States, 4Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
Spinal cord DTI is technically challenging, primarily due to the small size of the cord and physiological motion. We compare three different sequences to address these challenges: 1) single-shot EPI (ss-EPI), 2) multi-shot EPI with 2D navigation (IRIS), and 3) multi-shot EPI with reduced FOV (IRIS+iZOOM). Results indicate that IRIS alleviates geometric distortion present in ss-EPI. The combination of IRIS and iZOOM, however, provides the most consistent and least distorted diffusion-weighted images. These effects are clearly seen in the fractional anisotropy, axial diffusivity, and radial diffusivity maps, where exceptional contrast between gray and white matter are observed with IRIS+iZOOM.
Introduction
Diffusion tensor imaging (DTI) of the human spinal cord has significant potential in assessing the integrity of tissue microstructure, but has been clinically hampered due to several technical challenges. Single-shot echo planar imaging (ssh-EPI) is often used for DTI to acquire fast images, minimizing image degradation due to motion, but suffers from geometric distortions and signal losses, which is further exacerbated by high-resolution demands of spinal cord DTI. Alternatively, by acquiring a smaller subset of k-space after each excitation, multi-shot EPI (msh-EPI) can reduce bandwidth-related distortions. A challenge with msh-EPI, however, is that phase variations between shots must be corrected for. To this end, a 2D navigator echo is acquired to retrospectively compensate for phase changes. In this study, we apply msh-EPI with an image-reconstruction using image-space sampling function (IRIS)1 and compare it to a standard ssh-EPI acquisition. To further minimize image distortions, the echo train may be reduced by using reduced FOV methods.2 Therefore, we also demonstrate the utility and advantages of IRIS combined with iZOOM3 for diffusion tensor estimation in the in vivo spinal cord.Methods
Acquisition: Two healthy volunteers participated in this study under a protocol approved by in the institutional review board (1M/1F, 25-27 years old). Imaging was performed on a 3.0T whole body Philips Achieva scanner (Best, Netherlands). A two-channel body coil was used in multi-transmit mode for excitation and a 16-channel SENSE neurovascular coil was used for reception. Three sequences were acquired: 1) ssh-EPI, 2) msh-EPI with IRIS, 3) reduced FOV using iZOOM and msh-EPI with IRIS. For the first two sequences, the following parameters were used: FOV=140x140x30 mm3, resolution=1x1x5 mm3, SENSE (AP)=1.5, TR=3500 ms; the echo time was set to ‘shortest’ for all sequences, yielding a TE of 71 (ssh-EPI), 71 (IRIS) and 54 ms (IRIS+iZOOM). Diffusion was performed using 15 directions with a maximum b-factor of 750 s/mm2. For the IRIS+iZOOM sequence, all parameters remained the same except the FOV was reduced to 64x48x30 mm3, and thus the SENSE factor was reduced to 1. For fair comparison, scan averages were varied for each acquisition to keep scan times consistent, yielding 6, 3, and 3 averages for the ssh-EPI, IRIS, and IRIS+iZOOM acquisitions respectively.
Processing: Each diffusion-weighted acquisition was registered to a b=0 s/mm2 volume using the FLIRT package from FSL v5.0.2.1 (FMRIB, Oxford, UK). Diffusion tensor calculation was estimated with a nonlinear fit in Camino.4
Results
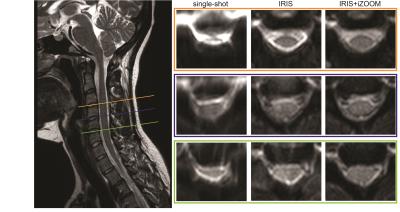
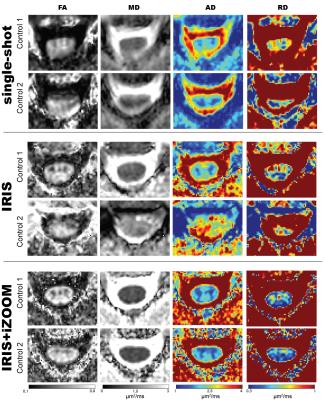
Figure 1 displays the non-diffusion weighted volume for each acquisition in a representative healthy control at three different anatomical levels (C3-4, C4-5, C5-6). The ssh-EPI shows significant spinal cord distortion, causing a piling of signal in the CSF, which is especially visible at the C3-4 level. Though the geometric distortion is alleviated with the IRIS acquisition, it is clear that the IRIS+iZOOM combination provides the most consistent and least distorted images. These artifacts propagate into the estimation of the diffusion tensor, as shown in the DTI-derived maps in Figure 2. For each acquisition, the fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) maps are shown for two different controls at C4. Of highest note, there is exceptional contrast between white and gray matter in the FA, AD, and RD maps for both controls using the IRIS+iZOOM acquisition, with the dorsal horns being clearly depicted. These internal spinal cord features are completely lost in the images derived from the single shot acquisition, and less visible in the IRIS acquisition, especially notable in control 2.Conclusion
While multi-shot with IRIS decreases geometric distortion and blurring effects in comparison to conventional ssh-EPI acquisitions, the combination of iZOOM with IRIS consistently provides high contrast of internal spinal cord features and minimal geometric distortions of the spinal cord. The use of this robust sequence has significant clinical implications in assessing the role of white matter integrity in neurodegenerative disease.Acknowledgements
No acknowledgement found.References
1. Jeong HK., et al. MRM. 2013; 69: 793-802.
2. Samson RS., et al. PloS One. 2016; 11.
3. Wu Z., et al. Proc ISMRM 2015, #953.
4. Cook, P.A., et al., Proc ISMRM 2006, #2759.
Figures