4670
Correlation of humanpapilloma virus status with quantitative perfusion/diffusion MRI parameters and metabolic 18F-FDG-PET parameters in oral cavity and oropharyngeal squamous cell carcinoma : Comparison between primary tumor lesions and metastatic lymph nodes1Radiology, Ajou University Medical Center, Suwon, Korea, Republic of, 2Nuclear medicine, Ajou University Medical Center, Suwon, Korea, Republic of
Synopsis
We evaluated association between perfusion/diffusion MRI parameters, metabolic 18F-FDG PET parameters and HPV status in not only primary oral cavity-oropharyngeal squamous cell carcinoma but also its metastatic lymph nodes. Any significant difference was not found in quantitative perfusion, diffusion, metabolic parameters of primary tumor between HPV-positive and HPV-negative groups. In case of metastatic lymph node, only metabolic parameters were significantly higher in HPV-positive group. In our study population, HPV status of primary OC-OPSCC and metastatic lymph nodes did not translate into different perfusion/diffusion parameters. Larger study population is needed to establish whether imaging parameters can represent the HPV status.
INTRODUCTION
The HPV associated oral cavity and oropharyngeal squamous cell carcinoma (OC-OPSCC) seems to be a distinct disease entity. Clinically, patients with HPV-positive tumor present mostly at younger age and advanced nodal stage, however, they respond more favorably to standard treatment and consequently show better survival compared to those with HPV-negative tumor.1, 2 This study was designed to investigate the differences in perfusion/diffusion MRI parameters and metabolic 18F-FDG PET parameters according to the presence of biologically active human papillomavirus (HPV) in OC-OPSCC. The correlation was evaluated in primary tumor site and metastatic lymph nodes, separatively.METHODS
41 patients with pathologically confirmed OC-OPSCC and underwent pretreatment MRI and 18F-FDG PET/CT were finally enrolled in this retrospective study. Among them 29 patients showed lymph nodes metastasis. The DCE-MR and diffusion images were post-processed by using commercially available software (nordicICE, NordicNEuroLab). Quantitative parameters (Ktrans, Kep, Ve, Vp, AUC60-area under the signal intensity-time curve at initial 60s) from DCE-MRI and ADC value from DWI were calculated within the manually placed ROI. A freehand ROI was plotted around the main tumor and the largest metastatic lymph node at the ipsilateral side of primary tumor site on every image slice. Maximum SUV (SUVmax) was measured for the entire tumor volume of interest. Mean SUV (SUVmean) and total lesion glycolysis (TLG = SUVmean x MTV, metabolic tumor volume) were calculated with the margin thresholds as 25%. Data normality was tested by the Kolmogorov-Smirnov test. Differences between HPV-positive and HPV-negative group were analyzed using χ2test (or Fisher’s exact test if appropriate) for categorical variables and t-test (or Mann–Whitney U test if appropriate) for continuous variables.RESULTS
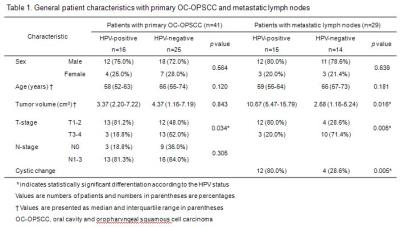
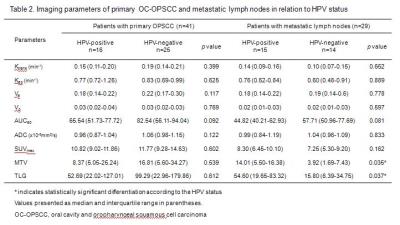
39.0% (16 patients) of primary tumors and 51.7% (15 patients) of metastatic lymph nodes showed HPV-positivity. Patients who had HPV-positive OC-OPSCC showed lower T stage (p=0.034), nevertheless they are more likely to present with N-positive disease. Metastatic lymph nodes in HPV-positive patients were more bulky (p=0.016) and showed cystic morphology more frequently (p=0.005) than those in HPV-negative patients (Table 1). The median values of DCE-MRI, DWI and 18F-FDG PET parameters according to HPV-status were presented in Table 2. Any significant difference was not found in quantitative perfusion, diffusion, metabolic parameters of primary tumor between HPV-positive and HPV-negative groups. In case of metastatic lymph node, only metabolic parameters (MTV, p=0.035; TLG, p=0.037) were significantly higher in HPV-positive group (Figure 2).DISCUSSION
There are some conflicting studies that analyzed relationship between perfusion/diffusion parameters, 18F-FDG PET parameters and HPV status. 3-10 In these reports, the correlations were analyzed in mainly primary tumor site and using only one kind of imaging parameters. Our study evaluated relationship between various imaging parameters and HPV status in same population simultaneously and analysis performed in only primary tumor site but also metastatic lymph nodes. Our study results were not fully compatible with previous studies that showed significant relationship between perfusion/diffusion parameters and HPV- positivity. These can be from different post processing methods for DCE-MRI and different DWI acquisition techniques.CONCLUSION
In our study population, HPV status of primary OC-OPSCC and metastatic lymph nodes did not translate into different perfusion and diffusion parameters. Larger study population and combination with histologic evaluation are needed to establish whether imaging parameters can represent the HPV status.Acknowledgements
No acknowledgement found.References
1. Marur S, D'Souza G, Westra WH, et al. HPV-associated head and neck cancer: a virus-related cancer epidemic. The Lancet Oncology 2010;11:781-789
2. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. The New England journal of medicine 2010;363:24-35
3. Choi YS, Park M, Kwon HJ, et al. Human Papillomavirus and Epidermal Growth Factor Receptor in Oral Cavity and Oropharyngeal Squamous Cell Carcinoma: Correlation With Dynamic Contrast-Enhanced MRI Parameters. American Journal of Roentgenology 2016;206:408-413
4. Jansen JFA, Carlson DL, Lu Y, et al. Correlation of a priori DCE-MRI and 1H-MRS data with molecular markers in neck nodal metastases: Initial analysis. Oral Oncology 2012;48:717-722
5. Schouten CS, de Graaf P, Bloemena E, et al. Quantitative Diffusion-Weighted MRI Parameters and Human Papillomavirus Status in Oropharyngeal Squamous Cell Carcinoma. American Journal of Neuroradiology 2015;36:763-767
6. Nakahira M, Saito N, Yamaguchi H, et al. Use of quantitative diffusion-weighted magnetic resonance imaging to predict human papilloma virus status in patients with oropharyngeal squamous cell carcinoma. European Archives of Oto-Rhino-Laryngology 2013;271:1219-1225
7. Driessen JP, van Bemmel AJM, van Kempen PMW, et al. Correlation of human papillomavirus status with apparent diffusion coefficient of diffusion-weighted MRI in head and neck squamous cell carcinomas. Head & Neck 2016;38:E613-E618
8. Joo Y-H, Yoo I-R, Cho K-J, et al. Preoperative18F-FDG PET/CT and high-risk HPV in patients with oropharyngeal squamous cell carcinoma. Head & Neck 2014;36:323-327
9. Schouten CS, Hakim S, Boellaard R, et al. Interaction of quantitative18F-FDG-PET-CT imaging parameters and human papillomavirus status in oropharyngeal squamous cell carcinoma. Head & Neck 2016;38:529-535
10. Cheng NM, Chang JT, Huang CG, et al. Prognostic value of pretreatment (1)(8)F-FDG PET/CT and human papillomavirus type 16 testing in locally advanced oropharyngeal squamous cell carcinoma. European journal of nuclear medicine and molecular imaging 2012;39:1673-1684
Figures