4664
The application of enhanced MRI with 3D-STIR-SPACE for Brachial Plexus lesions1MRI, The 1st affiliated hospital of Henan university of TCM, Zhengzhou, People's Republic of China, 2Chang Gung Memorial Hospital, Chiayi Branch, Taiwan
Synopsis
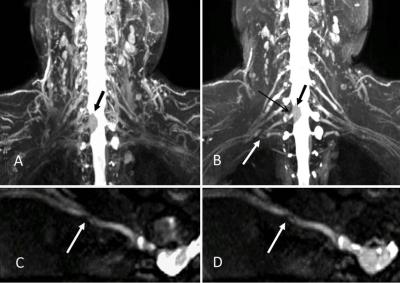
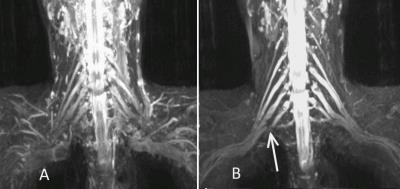
Imaging the brachial plexus is a challenge due to its complicated structure. The purpose of this study is to improve the diagnosis ability for brachial plexus 3D-STIR-SPACE technique with contrast agent administration. After contrast agent administration, signals of adjacent vessels were suppressed due to reduction in its T1 relaxation time which became similar to that of fat. The outlines of nerves would be clearer with respect to surrounding tissues.The image of 3D-STIR-SPACE technique with contrast agent was superior to that without contrast agent. It might be a better way to evaluate anatomies and pathologies of the brachial plexus.
Introduction
The pathologic conditions related with the brachial plexus include tumors, trauma, entrapment and irradiation. One of the current standard MRI protocols used in assessing the brachial plexus is 3D short inversion time inversion recovery sampling perfection with application optimized contrasts using different flip angle evolutions (3D-STIR SPACE) known for suppressing the signal from tissues with a short T1 relaxation time similar to fat. As a consequence, brachial plexus lesions could be nicely delineated by the maximum intensity projection (MIP) and multi-planar reformatting (MPR).In such cases, the outlines of brachial plexus would be inclined to be not clear due to overlapping with vessels. On the other hand, it is evidenced that T1 relaxation time of blood could be reduced as short as that of fat after contrast agent (Gd-DTPA) administration. Based on this framework, the 3D-T2-STIR images without and with contrast agent administration were compared to see if the image quality had been improved. The quantitative analyses would be achieved by rating the subjective diagnosis ability and evaluating the objective contrast ratio (CR) between the brachial plexus and the surrounding tissues.Methods
A total of 30 patients with brachial plexus diseases were recruited in this study (6 with brachial plexus injuries, 4 with neurogenic tumors, 1 with an intraspinal meningioma, 6 with tumors near brachial plexus and 13 with unremarkable findings). All images were performed on a 3 Tesla MRI system (Magnetom Trio, Siemens, Erlangen, Germany). The scan parameters were as follows: TR/TE= 4500/108 mc, flip angle=180°, echo train length=103, TI=220msec, slice thickness=1.1mm, slices oversampling=30%, slice per slab=40, field of view (FOV)=280mm×280mm, matrix size=256×256, number of acquisitions=1.4. The images were obtained both before and after the administration of contrast agent (Gd, Magnevist, Schering, Germany). The contrast ratio(CR), signal to noise ratio (SNR) and contrast to noise ratio(CNR) were measured by three neuro-radiologists. The t-test was used to assess the differences in CR between images without and with contrast agents. The intraclass correlation coefficient (ICC) was obtained between three observers. The Wilcoxon signed-rank test was used to test whether the diagnosis ability was improved after contrast agent. A P value of less than 0.05 was considered significant. .Results
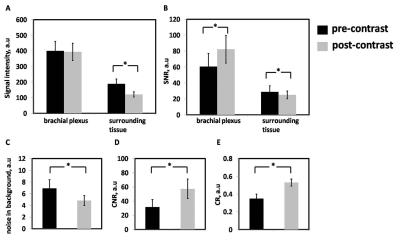
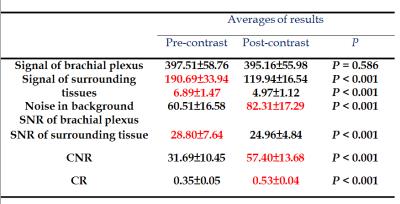
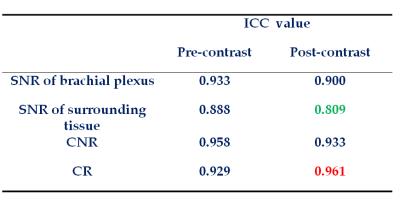
The signal intensity for brachial plexus without and with contrast agent were 397.51±58.76 and 395.16±55.98 and P value was 0.586, the difference of signal intensities was not significantly. For surrounding tissues, however, the signal intensities were significantly suppressed with the contrast agent (P<0.05); those without and with contrast agent were 190.69±33.94 and 119.94±16.54. In addition, images with contrast agent exhibited a higher CR (0.53 ± 0.04) than that without contrast agent (0.35 ±0.05). The higher CR also explained the improved diagnosis ability in images with contrast agent administration. The Wilcoxon sign-ranked test for signal intensities of brachial plexus between pre- and post-contrast MIP images showed p value was 0.586 and Z score was -5.45. The ICC value for post-contrast of SNR of brachial plexus was 0.99 and for post-contrast of SNR of surrounding tissue was 0.809.Discussion
This study provided evidences that with the help of contrast agent, diagnosis ability of 3D-STIR-SPACE images can be improved via suppressing signals of surrounding tissues and paraspinal vessels. This may be due to the fact that the signals of surrounding tissues and vessels were further suppressed. In the vicinity of the brachial plexus, there exist some vessels, including paraspinal vessels, subclavain veins, internal jugular veins and others. After a short period of time of TI, both nerves and blood in vessels are still hyperintense with respect to the fat and surrounding tissues because of their intrinsic MR properties. Under the circumstance of contrast agent administration, the T1 relaxation time of blood in vessels will be reduced while that of nerves will keep the same. It was achieved successfully the physical property of decreasing T1 relaxation time in vessels to improve the CR and the diagnosis ability. The advantages of the techniques was that they provide details of pathological lesions and anatomical structures, as well as clear outlines of the brachial plexus.Conclusion
In conclusion, the 3D-STIR-SPACE technique with contrast agent was superior to that without contrast agent. It might be a better way toevaluate the nerve anatomy and brachial plexus pathologies. These advantages would help to understand and make a detailed plan for surgery on brachial plexus in the future.Acknowledgements
No acknowledgement found.References
1. Vargas MI, Viallon M, Nguyen D, et al. New approaches in imaging of the brachial plexus. Eur J Radiol. 2010; 74:403-10.
2. Viallon M, Vargas MI, Jlassi H, et al. High-resolution and functional magnetic resonance imaging of the brachial plexus using an isotropic 3D T2 STIR (Short Term Inversion Recovery) SPACE sequence and diffusion tensor imaging. Eur Radiol. 2008;18:1018-23.
3. Kjelstrup T, Courivaud F, Klaastad O, et al. High-resolution MRI demonstrates detailed anatomy of the axillary brachial plexus. A pilot study. Acta Anaesthesiol Scand. 2012;56:914-9.
4. Van Es HW, Bollen TL, van Heesewijk HP. MRI of the brachial plexus: a pictorial review. Eur J Radiol. 2010;74:391-402. 6. Bowen BC, Pattany PM, Saraf-Lavi E, et al. The brachial plexus: normal anatomy, pathology, and MR imaging. Neuroimaging Clin N Am. 2004;14:59-85, vii-viii.
5. Lu H, Law M, Johnson G, et al. Novel approach to the measurement of absolute cerebral blood volume using vascular-space-occupancy magnetic resonance imaging. Magn Reson Med. 2005;54:1403-11.
6. Lichy MP, Wietek BM, Mugler JP 3rd, et al. Magnetic resonance imaging of the body trunk using a single-slab, 3-dimensional, T2-weighted turbo-spin-echo sequence with high sampling efficiency (SPACE) for high spatial resolution imaging: initial clinical experiences. Invest Radiol. 2005;40:754-60.
7. Gambarota G, Mekle R, Mlynarik V, et al. NMR properties of human median nerve at 3 T: proton density, T1, T2, and magnetization transfer. J Magn Reson Imaging. 2009;29:982-6.
8. Lu H, Clingman C, Golay X, et al. Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magn Reson Med. 2004;52:679-82.
9. Andreisek G, Crook DW, Burg D, et al. Peripheral neuropathies of the median, radial, and ulnar nerves: MR imaging features. Radiographics. 2006;26:1267-87.
10. Amrami KK, Port JD. Imaging the brachial plexus. Hand Clin. 2005;21:25-37.
Figures