4656
Inhibitory Motor Dysfunction in Parkinson’s Disease Subtypes1MRI, Shandong Medical Imaging Research Institute, Shandong University, Jinan, People's Republic of China, 2Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins university, Baltimore, MD, United States, 3MRI, Shandong Medical Imaging Research Institute, 4Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins university, Baltimore, MD, 5Shandong University, 6MRI, Philips Healthcare, Shanhai, People's Republic of China
Synopsis
This study was aimed to evaluate the differences between PD motor subtypes of GABA+ levels using MEGA-PRESS. PD patients were classified into PIGD and TD groups; sixteen healthy controls were recruited. All subjects underwent 3T MR examination including MEGA-PRESS. We found that GABA+ concentration was lower in PD compared with controls; furthermore, the TD group was lower than PIGD. In PD patients, the GABA+ levels were correlated with UPDRS scores. The results suggest that GABAergic dysfunction may play an important role in the pathogenesis of Parkinson’s disease. MEGA-PRESS provides a valuable examination method to discriminate between PD motor subtypes.
Objective
Parkinson’s disease (PD) is divided into two motor subtypes: postural instability gait difficulty (PIGD) and tremor-dominant (TD) subtypes. Increasing evidence has suggested that the GABAergic neurotransmitter system is involved in the pathogenesis of PD1, 2; however, to-date, MRS of GABA in PIGD and TD subtypes has not been performed. Thus, the aim of this study was to evaluate the differences between PD motor subtypes of GABA+ levels using MEGA-PRESS 3.Materials and Methods
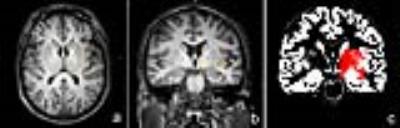
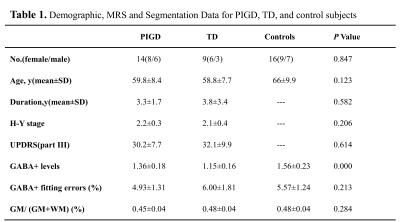
PD patients were classified into PIGD (n = 14) and TD groups (n = 9); sixteen age- and gender- matched healthy controls were recruited (Table 1). All subjects were right-handed, underwent 3T MR examination including MEGA-PRESS. GABA+ levels were quantified in the left basal ganglia (BG) using Gannet 4. Segmentation of T1-weighted images using SPM8 was also performed (Fig.1-2). Differences of GABA+ levels among three groups were analyzed using Analysis of Variance (ANOVA). The sensitivity, specificity, and accuracy of BG GABA+ levels for differentiating PIGD and TD were calculated. The relationship between GABA+ levels and unified Parkinson's disease rating scale (UPDRS) was also analyzed.Results
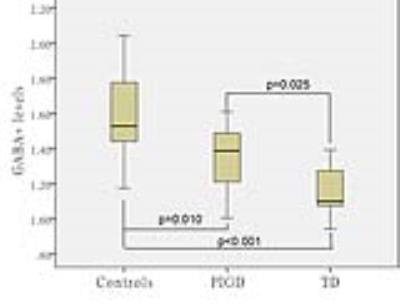
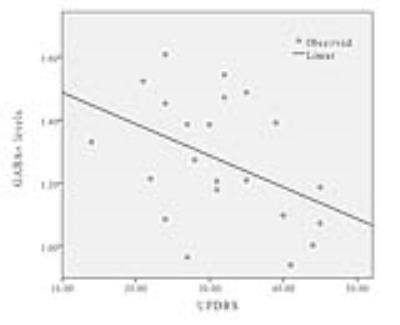
GABA+ levels were significantly lower in left BG regions of PD patients compared with healthy controls (P<0.001). In PD patients, the GABA+ concentration was lower in the TD group than PIGD group (p=0.025). No differences in voxel segmentation were observed. The sensitivity, specificity, and accuracy of BG GABA+ levels for differentiating PIGD and TD were 61.5%, 88.9% and 80.3% respectively (Fig.3). A significant negative correlation was found between GABA+ levels and UPDRS (r=-0.427, p=0.047) (Fig.4).
Discussion
Previous work 2 has shown altered GABA in PD. We further demonstrate significant differences in GABA+ levels between the major motor subtypes of PD and a correlation with UPDRS scores, indicating that altered GABA levels relate to symptom type and severity. Sensitivity/specificity/accuracy analysis suggests that MRS of GABA might be useful in differentiating the PD subtypes, and investigating their differing pathophysiology.Conclusion
Low BG GABA+ levels in PD patients suggest that GABAergic dysfunction may play an important role in the pathogenesis of Parkinson’s disease. MEGA-PRESS provides a valuable examination method to discriminate between PD motor subtypes.Acknowledgements
NoneReferences
1. Dharmadhikari S, Ma R, Yeh CL, et al. Striatal and thalamic GABA level concentrations play differential roles for the modulation of response selection processes by proprioceptive information. NeuroImage. 2015; 120:36-42.
2. Emir UE, Tuite PJ, Oz G. Elevated pontine and putamenal GABA levels in mild-moderate Parkinson disease detected by 7 tesla proton MRS. PloS one. 2012;7(1):e30918.
3. Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR in biomedicine. 1998;11(6):266-272.
4. Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. Journal of magnetic resonance imaging: JMRI. 2014;40(6):1445-1452.
Figures