4654
Implications of Obstructive Sleep Apnea on Global Cerebral Metabolic Rate of Oxygen1Department of Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Department of Neurology, University of Pennsylvania School of Medicine, Philadelphia, PA, United States, 3Division of Sleep Medicine, Department of Medicine, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Obstructive sleep apnea (OSA) is a chronic disorder caused by intermittent obstruction of the upper airways during sleep. Neurocognitive deficits in this population have previously been associated with altered brain metabolism. In fact, a recent pilot study suggests that global cerebral metabolic rate of oxygen (CMRO2) may be a potential marker of oxygen metabolic dysfunction in this population. Here, we present preliminary results from an ongoing study designed to quantify CMRO2 in subjects with OSA and matched controls. Initial results suggest that apneics have overall lower baseline CMRO2 and increased response to volitional apnea, a paradigm to mimic spontaneous apneas.
PURPOSE
Obstructive sleep apnea (OSA) is a common chronic disorder of the upper airways preventing patency during sleep, therefore causing periodic desaturation of arterial blood. OSA predisposes subjects to cardiac and neurovascular disease and is associated neurocognitive deficiencies. It has therefore been hypothesized that OSA results in altered oxygen metabolism of the brain.1 Here we present initial results from an ongoing longitudinal study designed to quantify global cerebral metabolic rate of oxygen (CMRO2) in subjects with OSA and BMI- and age-matched controls, both at rest and during volitional apnea, the latter being hypothesized to mimic nocturnal spontaneous apneas.METHODS
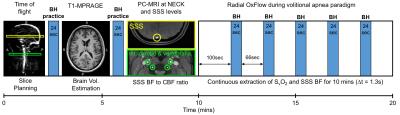
Knowledge of the arteriovenous difference in oxygen saturation (SaO2-SvO2) and blood flow enables calculation of CMRO2 consumption from Fick’s law CMRO2=Ca·CBF·(SaO2-SvO2). Here, Ca is the hemoglobin O2 carrying capacity and CBF is cerebral blood flow. SvO2 is quantified based on the intrinsic susceptibility of deoxygenated hemoglobin, while SaO2 is measured by pulsed oximetry, and total CBF is estimated via MRI phase-contrast in the arteries supplying the brain. The MRI protocol and paradigm is shown in Fig. 1. Data were acquired at 3T (Prisma Siemens); here, a technique for rapid quantification of CMRO2 consisting of a three-echo spoiled gradient-echo sequence with velocity-encoding gradients and radial encoding was used.2 Imaging parameters: TR/TE1/TE2=19/5.5/13.5ms, FOV=240×240mm2, voxel size=1×1×5mm3, FA=15°, VENC=76.42cm/s; views per image 610 and temporal footprint of 23.18s. Data were updated every 34 views resulting in an effective temporal resolution of 1.29s. The sequence was run continuously for 10 minutes thereby creating a total of 4 images in the time series. Processing yielded SvO2 from the tissue-blood phase difference2 in the superior sagittal sinus (SSS), along with CBF at the same location. SSS flow rates were then upscaled via a calibration scan in which velocity was measured once at baseline in internal carotid and vertebral arteries (since the SSS drains the cerebral cortex only). CBF was normalized per 100g of brain tissue, estimated from a MP-RAGE-based measurement of brain volume. The volitional apnea paradigm protocol consisted of five 24s breath-holds (BH) with 66s of recovery, during 10 minutes of continuous data acquisition (Fig. 1). Subjects were cued visually and acoustically to breath in, breathe out and hold 6, 3 and 0s before the start of each BH, and to breathe normally after 24s of BH. The five successive breath-hold cycles yielding SaO2, SvO and CBF were then averaged into a single 90s block, and CMRO2 computed as in Rodgers et al.1 Hemoglobin levels were obtained from capillary blood obtained via fingerprick. Differences in oxygen metabolism between OSA subjects and control groups were examined using unpaired t-tests given that at the present stage no one-on-one matching of controls and OSA subjects existed.RESULTS
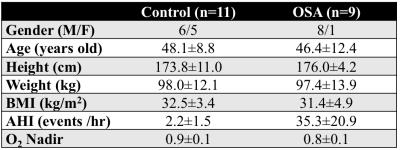
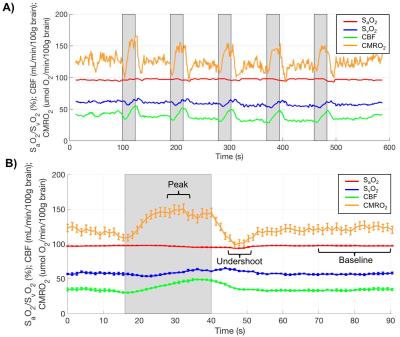
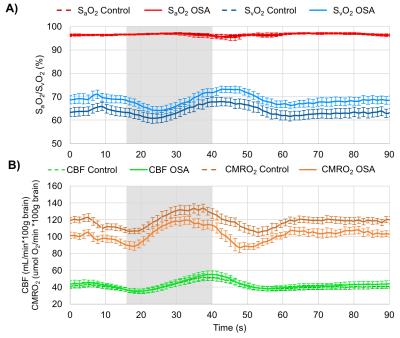
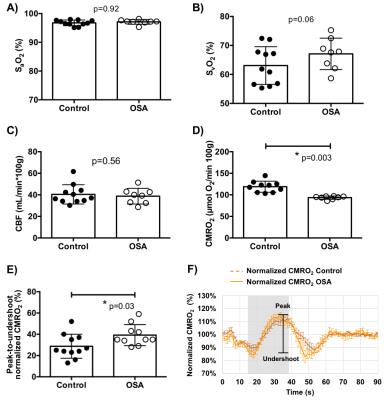
Ten OSA patients and 11 matched controls have been studied so far (see Table for demographics). An example dataset throughout the duration of the time series is shown in Fig. 2. Averaged time-courses in both groups for all variables are shown in Fig. 3. None of the measured baseline parameters (SaO2, SvO2, CBF) were different between OSA and control groups (p>0.05, Fig. 4A-C). However, CMRO2 was significantly lower in OSA subjects at baseline (118.9±12.9 vs 93.6±3.8 μmol O2/100g/min, p=0.003, Fig. 4D). Peak CMRO2 during volitional apnea was lower as well (134.0±16.9 vs 121.3±10.9 μmol O2/100g/min, p=0.02) as was the undershoot (100.53±14.0 vs 80.3±12.5 μmol O2/100g/min, p=0.002). The baseline-to-peak fractional change in CMRO2 was suggestive of being higher in apneics, but did not reach statistical significance (13.2±7.6% vs 16.4±7.2%, p=0.3), but the peak-to-undershoot response was significantly greater in apneics than in controls (28.7±11.3%, 39.2±10.1%, p=0.03, Figs. 4E-F).DISCUSSION AND CONCLUSIONs
Results presented here parallel the normal physiological response to hypercapnia and hypoxia during which oxygen delivery is limited to prevent hypoxic brain injury.3 Although the volitional apnea paradigm may not accurately replicate the physiology during spontaneous OSA episodes, our work effectively demonstrates differences in oxygen metabolism between people with and without OSA. A prior pilot study from the authors’ lab suggests that CMRO2 may be a more sensitive marker of oxygen metabolic dysfunction than CBF and oxygen extraction.1 Differences between the present study and the one by Rodgers et al are: (1) increased temporal resolution made possible by the radial encoding strategy used, (2) enrollment of BMI and age-matched controls, and (3) duration of the BH period. Although the number of participants in our study is small, the data suggest that the lower baseline CMRO2 in apneics is driven mostly by SvO2 even though neither CBF nor SvO2 in themselves were significantly different between OSA subjects and controls.Acknowledgements
NIH Grant RO1 HL122754, RO1 HL109545.References
1. Rodgers ZB, Leinwand SE, Keenan BT, Kini LG, Schwab RJ, Wehrli FW. Cerebral metabolic rate of oxygen in obstructive sleep apnea at rest and in response to breath-hold challenge. J Cereb Blood Flow Metab. 2016;36:755-67.
2. Rodgers ZB, Jain V, Englund EK, Langham MC, Wehrli FW. High temporal resolution MRI quantification of global cerebral metabolic rate of oxygen consumption in response to apneic challenge. J Cereb Blood Flow Metab. 2013;33:1514-22.
3. Gooden BA. Mechanism of the human diving response. Integr Physiol Behav Sci. 1994;29:6-16.
Figures