4650
Effects of astrocytic Nrf2 activation on recently-identified 13C and 1H MRS flux-based biomarkers of mitochondrial energetics and neurotransmitter cycling in Huntington's Disease1Department of Psychiatry, Yale University School of Medicine, New Haven, CT, United States, 2Department of Psychiatry, Yale University School of Medicine, 3Department of Radiology and Biomedical Imaging, Yale University School of Medicine, New Haven, United States, 4Department of Radiology and Biomedical Imaging, Yale University School of Medicine, New Haven, CT, United States, 5School of Pharmacy, School of Pharmacy, University of Wisconsin, Madison, WI, United States, 6CHDI Management, CHDI Management/CHDI Foundation, Los Angeles, United States
Synopsis
Alterations in brain glucose and energy metabolism is observed in Huntington’s Disease (HD) and HD animal models. 1H-[13C]-MRS can be readily adapted to measure metabolic pathway flux by use of 13C-labeled substrates. In this study we assessed whether activation of the astroglial Nrf2-ARE pathway in the R6/2 mouse model of HD, which has shown therapeutic potential in HD animal models, can reverse the reduction in 13C labeling seen previously in R6/2 mice. In cortex and striatum, astroglial Nrf2 activation led to increased amino acid 13C labeling, suggesting a degree of improvement in mitochondrial and neurotransmitter fluxes in the R6/2 mice.
PURPOSE: Alterations in brain energy metabolism, including reduced glucose utilization and mitochondrial respiration, is observed in Huntington’s Disease (HD) and HD animal models (1). Despite our detailed knowledge of the mutated gene and its poly-glutaminated (poly-Q) protein product, mutant huntingtin (mHtt), the mechanism underlying pathology and progression of HD remains enigmatic. There is an acute compelling need to apply new experimental tools to broaden the search for the disease mechanism and identify potential therapeutic targets. Magnetic Resonance Spectroscopy (MRS) offers one such tool, which has been applied recently in HD afflicted individuals and HD animal models (1,2). MRS can be readily adapted to measure metabolic pathway flux by use of 13C-labeled substrates. Using this approach we recently found significant and early reduction in neuronal TCA cycle and neurotransmitter cycling in the R6/2 mouse model of HD (3), suggesting their potential use of the 13C flux measures as biomarkers of pathophysiology and treatment response. In this study we utilized the 13C MRS approach to assess whether the HD-related changes in 13C flux measures observed previously in the R6/2 mouse can be reversed by upregulation of the Nrf2-ARE pathway in R6/2 mice, which has shown promise as a therapy in animal models of HD and other neurological diseases.
METHODS: R6/2 and wildtype mice overexpressing astroglial GFAP-Nrf2 were obtained from Drs. Jeffrey Johnson and Delinda Johnson, University of Wisconsin School of Pharmacy and studied at 9 to 10 postnatal weeks of age. Study groups consisted of the four genotypes, +/+ (+R6/2/+GAFP-Nrf2), -/+ (-R6/2/+GFAP-Nrf2), +/- (+R6/2/-GFAP-Nrf2) and -/- (-R6/2/-GFAP-Nrf2). All mice were allowed to recover for one week after surgery to place a jugular vein catheter, before infusion of the 13C-labeled substrate. Mice were infused with [1,6-13C2]glucose (8 min), rapidly raising the respective concentrations and 13C-enrichments to high and constant values (4,5). At the appropriate times, mice were quickly sedated (<30s) with isoflurane, loaded into a specialized holder and euthanized by focused-beam microwave irradiation, arresting brain metabolism in <1 sec (6). The brain was removed and dissected into cortex and striatum. Brain tissues were extracted using ethanol with [2-13C]glycine added as an internal concentration standard (5). The concentration and 13C enrichment of amino acids in the extract were determined using 1H-[13C] MRS at 11.74T (Bruker AVANCE NMR spectrometer) (7).
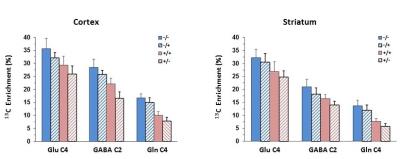
RESULTS: In cortex of R6/2 (+/-) mice, highly significant reductions in 13C labeling was seen in glutamate-C4 (↓27%, P<0.000), GABA-C2 (↓42%, P<0.000) and glutamine-C4 (↓53%, P<0.000) compared to control (-/-) mice. GFAP-Nrf2 overexpression in R6/2 mice (+/+), as compared to R6/2 (+/-) mice, led to higher 13C labeling in GABA-C2 (↑33%, P<0.000) and glutamine-C4 (↑26%, P<0.02), and insignificant increase in glutamate-C4 (↑13%, P=0.1). Overexpression of GFAP-Nrf2 alone (-/+), as compared to control (-/-) mice, led to ~10% lower 13C labeling in glutamate-C4 (P<0.03), GABA-C2 (P<0.04) and glutamine-C4 (P=0.055). In striatum of R6/2 (+/-) mice, highly significant reductions in 13C labeling was seen in glutamate-C4 (↓23%, P<0.000), GABA-C2 (↓34%, P<0.000) and glutamine-C4 (↓58%, P<0.000) compared to control (-/-) mice. GFAP-Nrf2 overexpression in R6/2 mice (+/+), as compared to R6/2 (+/-) mice, led to higher 13C labeling in GABA-C2 (↑18%, P<0.01) and glutamine-C4 (↑37%, P<0.01), and insignificant increase in glutamate-C4 (↑9%, P>0.5). Overexpression of GFAP-Nrf2 alone (-/+), as compared to control (-/-) mice, led to insignificant reductions in 13C labeling in glutamate-C4 (↓5%, P>0.6) and glutamine-C4 (↓12%, P>0.1), although the reduction in GABA-C2 (↓14%, P<0.05) reached statistical significance.
CONCLUSIONS: In both brain regions (cortex and striatum), R6/2 mice showed large reductions in 13C labeling of amino acids, consistent with our previous findings (3). Overexpression of Nrf2 in astrocytes of R6/2 mice lead to partial recovery of the deficit in 13C labeling of glutamate-C4, GABA-C2 and glutamine-C4 seen in untreated R6/2 mice. Our findings, though preliminary, suggest that increased expression of Nrf2 in astrocytes can reduce HD-associated reductions in mitochondrial and neurotransmitter fluxes seen in R6/2 mice.
Acknowledgements
This study was supported by the CHDI Foundation, Inc.References
1.Zacharoff et al. (2012) JCBFM 32:502-14.
2. Heikkinen et al. (2012) PLoS One 7(12):e50717.
3. Chowdhury et al. (2014) Abstract, ISMRM-ESMRMB, Milan, Italy.
4. Chowdhury et al. (2007) J Neurochem. 103:2077-2091.
5. Chowdhury et al. (2007) JCBFM 27:1895-907.
6. Banasr et al. (2010) Mol Psychiatry 15:501-11.
7. Chowdhury et al. (2012) Biol Psychiatry. 71(11):1022-5.
Figures