4649
QSM meets MRS: The influence of subcortical iron on glutamatergic neurotransmission in a movement disorder population1Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 2Department of Psychiatry, Social Psychiatry and Psychotherpay, Hannover Medical School, Hannover, Germany, 3Diagnostic Imaging, Magnetic Resonance, Research & Development, Siemens Healthcare GmbH, Erlangen, Germany, 4Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 5Douglas Mental Health University Institute and Department of Psychiatry, McGill University, Montreal, QC, Canada
Synopsis
We use a combination of Quantitative Susceptibility Mapping and 1H-MRS to examine the role of iron and its association with glutamatergic signalling in Gilles de la Tourette syndrome (GTS). GTS is a neurodevelopmental movement disorder with abnormalities in the neurotransmission of dopamine and GABA and, as shown more recently, also in subcortical glutamate (Glu) and glutamine (Gln). In this work, we observed that GTS patients exhibit reductions in cerebral iron levels and report a general association between iron and the Gln:Glu ratio. This work provides a good example of utilizing multi-modal neuroimaging methods to interrogate pathophyiology at multiple scales.
INTRODUCTION
Iron is a trace element that is essential to the vitality of an organism, as it is ideally suited for the catalysis of many biochemical reactions due to its ability to transition between two thermodynamically stable oxidation states. Within the brain, non-heme iron is abundant in subcortical nuclei, overlapping with regions that contain large proportions of dopamine, GABA and glutamate (Glu)1. The atypical homeostasis of iron during development may influence the biochemical mechanisms that sustain typical subcortical neurochemical signalling2,3, providing a possible biological basis for deficits exhibited by varied neuropsychiatric and movement disorders4,5. Gilles de la Tourette Syndrome (GTS) is an example of a neurodevelopmental movement disorder with reported reductions in serum ferritin levels6–8 and abnormalities in the neurotransmission of dopamine and GABA9. It was recently shown that GTS patients also exhibit reductions in striatal Glu and glutamine (Gln)10, indicating possible abnormalities in GABA-Glu-Gln cycling, which may lead to alterations in the spatio-temporal dynamics of excitatory, inhibitory and modulatory subcortical neurochemical signalling. Along this line, a comprehensive body of literature has indicated that iron deficiency alters the neurotransmission of dopamine and GABA by influencing the mechanisms involved in receptor function and neurotransmitter synthesis/transport3. Here, we used a combination of Quantitative Susceptibility Mapping (QSM) and Magnetic Resonance Spectroscopy (1H-MRS) to investigate (a) whether GTS patients exhibit alterations in iron metabolism, and (b) whether iron levels exhibit a general influence on the Gln:Glu ratio.METHODS
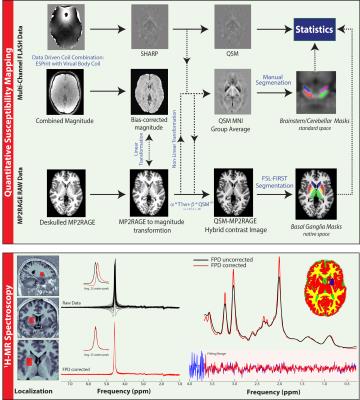
QSM, 1H-MRS and MP2RAGE11 data were acquired from 28 GTS patients and 22 age/gender matched healthy controls on a 3T Siemens MAGNETOM Verio using a 32-channel head coil. A 10ml blood sample was also collected from each subject for the quantitation of serum Ferritin. Susceptibility-weighted data were acquired using FLASH with =30ms; =17ms; 256x256 matrix; flip-angle=13°; 0.8mm isotropic nominal resolution. High-quality phase maps were reconstructed using an automated, data-driven coil combination method12,13. QSM images were computed using the superfast dipole inversion approach via SHARP bias removal and TKD susceptibility calculation14, with referencing to lateral ventricle CSF15 (Figure 1). An MP2RAGE-QSM hybrid contrast image was created to yield more robust/accurate segmentations of basal ganglia nuclei via FSL-FIRST16. Brainstem and cerebellar nuclei were mapped onto native QSM space by non-linear transformation of masks that were carefully delineated on a QSM group-average template. Median susceptibility values were extracted from each ROI and Wilcoxon rank-sum tests were used to assess group differences. Motion-affected data were removed using a multivariate robust Mahalanobis distance framework based on two structural image quality indices17,18. 1H-MRS data were acquired from a left striatal voxel (TE= 30ms, TR=3000ms, nav=128) employing automated voxel localization; frequency and phase-drift correction; and LCModel absolute metabolite quantitation with consideration of within-voxel compartmentation10. A multiple linear regression model accounting for age, gender and two indices of image quality was used to examine the relationship of susceptibility with Ferritin and the Gln:Glu ratio. The variance inflation factor (VIF) was used to assess multi-collinearity between predictor variables.RESULTS
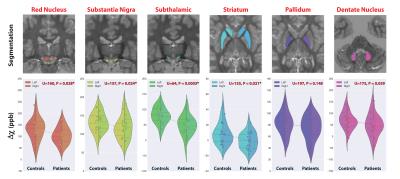
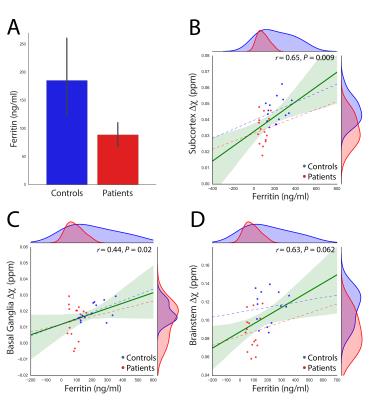
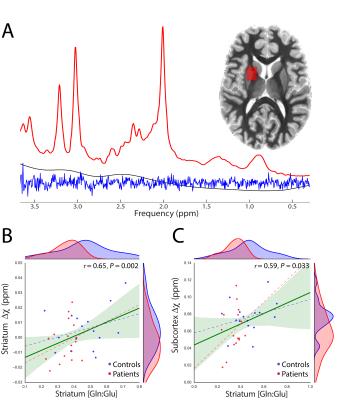
We report significant reductions in the susceptibility of brainstem (p=0.001), basal ganglia (p=0.04) and all subcortical (p=0.0007) nuclei of GTS patients in comparison to controls. These effects were mainly driven by significant reductions of susceptibility in the subthalamic nucleus, substantia nigra, striatum, and red nucleus (Figure 2). Subcortical susceptibility reductions were mirrored by significant reductions in serum Ferritin (Welch’s test, p=0.011; Figure 3). Correlation analyses in the whole sample revealed significant associations between Ferritin and susceptibility in all subcortical (R=0.65, p=0.009) and basal-ganglia (R=0.44,p=0.02) nuclei. Significant associations were also observed between striatal Gln:Glu and susceptibility in the striatum (R=0.65, p=0.002) and all subcortical nuclei (R=0.59, p=0.033). All correlations exhibited an approximate VIF of 1.5 indicating little to no multi-collinearity between predictor variables.DISCUSSION
Our results indicate that GTS patients exhibit abnormalities in cerebral iron homeostasis; and provide in-vivo evidence for a relationship between iron metabolism and glutamatergic neurotransmission. This result is supported by previous work indicating that gestational and lactational iron deficiency suppresses striatal19 and hippocampal20 glutamatergic neurotransmission, in addition to influencing enzymes involved in GABA-Glu-Gln cycling21–23. As iron is involved in multiple biochemical pathways that regulate neurochemical signalling, alterations of its hemostasis in GTS provides new clues on the biological basis of the observed disruptions in excitatory, inhibitory and modulatory signalling. Although GTS is considered a polygenic hereditary disorder with multiple affected systems, it is plausible to suggest that iron holds an important role in the pathophysiology of GTS, as its deficiency has been related to a neurochemically driven influence on the acquisition motor and behavioural functions during development5.Acknowledgements
This work was funded by the FP7 Marie Curie Actions of the European Commission (“TS-EUROTRAIN FP7-PEOPLE-2012-ITN, Grant No. 316978”) and, in part, by the Helmholtz Alliance ("ICEMED: Imaging and Curing EnvironmentalMetabolic Diseases”).References
1. Hill JM. The distribution of iron in the brain. In: Brain Iron: Neurochemical and Behavioural Aspects. Taylor and Francis London; 1988:1-24.
2. Youdim MBH, Ben-Shachar D, Yehuda S, Levitsky DA, Hill JM. Putative biological mechanisms of the effect of iron deficiency on brain biochemistry and behavior. Am. J. Clin. Nutr. 1989;50:607-617.
3. Bianco L, Unger E, Beard J. Iron Deficiency and Neuropharmacology. In: Yehuda S, Mostofsky DI, eds. Iron Deficiency and Overload: From Basic Biology to Clinical Medicine. Totowa, NJ: Humana Press; 2010:141-158.
4. Yehuda S. Neurochemical basis of behavioral effects of brain iron deficiency in anemia. Brain, Behav. iron infant diet 1990:63–81.
5. Beard JL, Connor JR. Iron status and neural functioning. Annu. Rev. Nutr. 2003;23:41-58.
6. Gorman DA, Zhu H, Ph D, Anderson GM, Davies M, Peterson BS. Ferritin Levels and Their Association With Regional Brain Volumes in Tourette’s Syndrome. 2006:1264-1272.
7. Cortese S, Lecendreux M, Bernardina BD, Mouren MC, Sbarbati A, Konofal E. Attention-deficit/hyperactivity disorder, Tourette’s syndrome, and restless legs syndrome: the iron hypothesis. Med. Hypotheses 2008;70(6):1128-32.
8. Peterson
BS, Gore JC, Riddle MA, Cohen DJ, Leckman JF. Abnormal magnetic resonance
imaging T2 relaxation time asymmetries in Tourette’s syndrome. Psychiatry
Res. 1994;55(4):205-21.
9. Singer H. The Neurochemistry of Tourette Syndrome. In: Martino D, Leckman JF, eds. Tourette Syndrome. Oxford University Press; 2013:276-297.
10. Kanaan A, Gerasch S, Garcia-Garcia I, et al. Pathological glutamatergic neurotranmission in Gilles de la Tourette Syndrome. Brain 2017. doi:10.1093/brain/aww285.
11. Streitbürger
DP, Pampel A, Krueger G, et al. Impact of image acquisition on
voxel-based-morphometry investigations of age-related structural brain changes.
Neuroimage 2014;87:170-182.
12. Uecker M, Lai P, Murphy MJ, et al. ESPIRiT - An eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn. Reson. Med. 2014;71(3):990-1001.
13. Bilgic B, Polimeni JR, Wald LL, Setsompop K. Automated tissue phase and QSM estimation from multichannel data. In: Proceedings of the 24th Annual Meeting of ISMRM; 2849; 2016.
14.Schweser
F, Deistung A, Sommer K, Reichenbach JR. Toward online reconstruction of
quantitative susceptibility maps: Superfast dipole inversion. Magn. Reson.
Med. 2013;69(6):1582-1594.
15. Straub S,
Schneider TM, Emmerich J, et al. Suitable reference tissues for quantitative
susceptibility mapping of the brain. In: Proceedings of the 24th Annual Meeting of ISMRM; 0035; 2016
16. Feng X, Deistung A, Schweser F, Guellmar D, Reichenbach JR. Investigation of brain segmentation with FIRST by using different hybrid contrasts and registrations. In: Proceedings of the 23rd Annual Meeting of ISMRM; 3497; 2015.
17. Mortamet B, Bernstein MA, Jack CR, et al. Automatic quality assessment in structural brain magnetic resonance imaging. Magn. Reson. Med. 2009;62(2):365-372.
18. Atkinson
D, Hill DL, Stoyle PN, Summers PE, Keevil SF. Automatic correction of motion
artifacts in magnetic resonance images using an entropy focus criterion. IEEE
Trans. Med. Imaging 1997;16(6):903-10.
19. Ward KL, Tkac I, Jing Y, et al. Gestational and lactational iron deficiency alters the developing striatal metabolome and associated behaviors in young rats. J. Nutr. 2007;137(4):1043-1049.
20. Rao R, Tkac I, Townsend EL, Gruetter R, Georgieff MK. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J. Nutr. 2003;133(10):3215-3221.
21. Erikson KM, Shihabi ZK, Aschner JL, Aschner M. Manganese accumulates in iron-deficient rat brain regions in a heterogeneous fashion and is associated with neurochemical alterations. Biol. Trace Elem. Res. 2002;87(1-3):143-156.
22. Ill AM, Mitchell TR, Neely EB, Connor JR. Metabolic analysis of mouse brains that have compromised iron storage. Metab. Brain Dis. 2006;21(2-3):77-87.
23. Taneja V,
Mishra K, Agarwal KN. Effect of early iron deficiency in rat on the
gamma-aminobutyric acid shunt in brain. J Neurochem
1986;46(6):1670-1674.
Figures