4645
Dependence of degree of motor impairment on the association between motor performance and sensorimotor GABA level in relapsing remitting multiple sclerosis1Imaging Institute, Cleveland Clinic, Cleveland, OH, United States, 2Neurological Institute, Cleveland Clinic, Cleveland, OH, United States
Synopsis
Gamma aminobutyric acid (GABA), a major inhibitory neurotransmitter, has been implicated as a metabolic marker of multiple sclerosis (MS). Previously it has been shown that sensorimotor cortex GABA level is higher in relapsing remitting MS (RRMS) patients with poorer motor performance. In this study, the association between cortical GABA level and motor performance, as measured by 9-hole peg test score, has been studied for groups of patients with RRMS with different degrees of motor impairment. The results suggest that cortical GABA has more involvement in motor performance in early stage of RRMS or in less impaired patients.
Purpose
The major inhibitory neurotransmitter GABA has recently been suggested to be a biomarker of multiple sclerosis (MS)1,2. Studying correlation between 9 hole peg test scores (9HPT), a measure of motor performance) and sensorimotor cortex GABA level [GABA], motor impairment has been reported to be associated with higher [GABA] in relapsing remitting MS (RRMS)3 and lower GABA in secondary progressive (SPMS)2. This difference prompts to look for association of GABA in different levels of motor impairment. Correlation of [GABA] and 9HPT of 2 groups of RRMS patients (with 9HPT Z score < 0 and > 0) were investigated in this study.Methods
MR scans were performed using a 3 Tesla Siemens whole body Tim-Trio scanner (Erlangen, Germany) under an Institutional Review Board approved protocol. RRMS patients were scanned with a MEGA-PRESS sequence4 (voxel size = 2×2×2 cm3, TR=2700 ms, TE=68 ms, number of averages = 96 ON and 96 OFF, editing pulse frequency = 1.9 (ON) /1.5 (OFF) ppm to reduce macromolecule contamination). A voxel at the motor cortex was selected prior to the spectroscopy scan from the area of maximum activation (Siemens Neuro3D program) following an fMRI scan in which each subject performed bilateral finger tapping in a block interleaved 32 second ON and 32 second OFF pattern. After discarding motion corrupted data as determined by interleaved water signal navigator signal or residual water signal3 a total of 24 RRMS patients’ (Age: 48±8 y, 6 M) data were used for analyses. PRESS scans with and without water suppression were performed for absolute quantification following the editing scans. For absolute quantification, the gray matter, white matter and CSF contribution to the voxel composition was performed by using FAST5 of FSL library6 with the anatomical 3D MPRAGE as the base image, and applying a mask at the voxel location. MRUI software was used for spectroscopy data analysis7. Absolute [GABA] was determined by taking the product of [GABA]/[Cr] (from MEGA-PRESS) and [Cr] using internal water reference (from a PRESS scan run during the same session)1. For 9HPT, the time taken by a subject to pick up and place 9 dowels in 9 holes was recorded8. The Z score for 9HPT score for each subject was calculated with methods delineated by the National Multiple Sclerosis Society Clinical Outcomes Assessment Task Force based on a pool of data involving a total of 4715 patients.9-11 Next, correlation coefficients of [GABA] and 9HPT scores (Z scored) (N=24) were calculated. In addition, the subjects were divided into 2 groups: (i) 9HPT<0 (N=9; Age: 48±8 y, 3 M), and (ii) 9HPT>0 (N=15; Age: 51±5 y, 3 M), and GABA - 9HPT correlation coefficient in each group was calculated.Results and Discussion
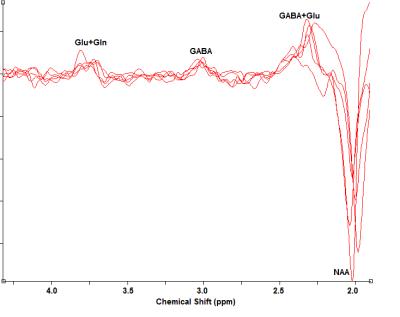
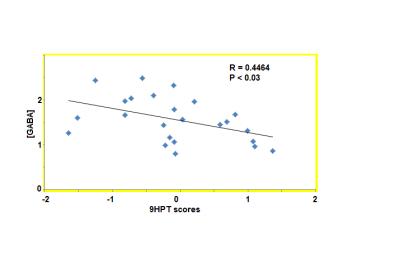
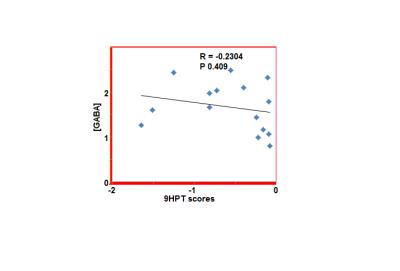
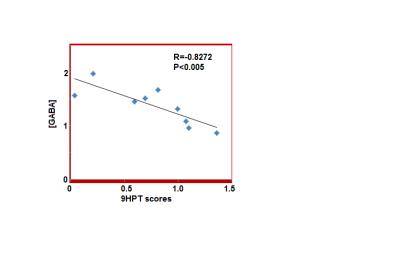
The 9HPT score and [GABA] for all subjects and the 2 groups (9HPT<0 and 9HPT>0) are shown in Table 1. Representative spectra from 5 subjects are shown in Fig. 1. The 9HPT scores between the 2 groups (Z<0 and Z>0) are significantly different (P < 0.0001 from unpaired t-test), which ensured a significant difference in motor performance between the 2 groups. Unpaired t-test showed no significant difference in [GABA] between the 2 groups. [GABA] – 9HPT correlation was significant (P<0.05) in all subjects combined group, not significant in 9HPT<0 group, and strongly significant (P<0.005) in 9HPT>0 group (Fig. 2-4). The correlation coefficients between the 2 groups are significantly different (P<0.05). Inverse correlation between [GABA] and 9HPT has previously been reported in RRMS3 suggesting a role of GABA in the disease process and a potential biomarker of MS. This study implies that GABA plays more significant role in motor functioning and thus the disease process in early stage of RRMS, i.e., in patients with less impairment (9HPT>0). On the other hand our data suggest that GABA does not play a significant role in more impaired patients (9HPT<0) with RRMS. It should be pointed out that the direct [GABA]-9HPT correlation in contrast with the inverse correlations reported here could be a result of (i) the GABA level reported in the secondary progressive MS study including contribution from co-edited macromolecule, and (ii) loss of compensatory adaptation process in secondary progressive MS.12Conclusion
Higher sensorimotor cortex [GABA] was observed in motor cortex of MS patients with more motor impairment. The correlation was significantly more in patients with less motor impairment (9HPT>0) than in more impaired patients (9HPT<0). Cortical GABA is suggested to have more involvement in motor performance in early stage of RRMS or in less impaired patients.Acknowledgements
National Institutes of Health, National Multiple Sclerosis Society, Research Programs Committee Cleveland Clinic, Siemens Medical Solutions.References
1. Bhattacharyya PK, Phillips MD, Stone LA, Lowe MJ. In vivo magnetic resonance spectroscopy measurement of gray-matter and white-matter gamma-aminobutyric acid concentration in sensorimotor cortex using a motion-controlled MEGA point-resolved spectroscopy sequence. Magn Reson Imaging. 2011;29(3):374-379.
2. Cawley N, Solanky BS, Muhlert N, Tur C, Edden RA, Wheeler-Kingshott CA, Miller DH, Thompson AJ, Ciccarelli O. Reduced gamma-aminobutyric acid concentration is associated with physical disability in progressive multiple sclerosis. Brain. 2015;138(Pt 9):2584-2595.
3. Bhattacharyya PK, Phillips MD, Stone LA, Bermel RA, Lowe MJ. Sensorimotor cortex gamma-aminobutyric acid concentration correlates with impaired performance in patients with MS. AJNR Am J Neuroradiol. 2013;34(9):1733-1739.
4. Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11(6):266-272.
5. Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45-57.
6. Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208-219.
7. http://www.mrui.uab.es/mrui/.
8. Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther. 1985;39(6):386-391.
9. Cutter GR, Baier ML, Rudick RA, Cookfair DL, Fischer JS, Petkau J, Syndulko K, Weinshenker BG, Antel JP, Confavreux C, Ellison GW, Lublin F, Miller AE, Rao SM, Reingold S, Thompson A, Willoughby E. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain. 1999;122 ( Pt 5):871-882.
10. Rudick R, Antel J, Confavreux C, Cutter G, Ellison G, Fischer J, Lublin F, Miller A, Petkau J, Rao S, Reingold S, Syndulko K, Thompson A, Wallenberg J, Weinshenker B, Willoughby E. Recommendations from the National Multiple Sclerosis Society Clinical Outcomes Assessment Task Force. Ann Neurol. 1997;42(3):379-382.
11. Fischer JS, Rudick RA, Cutter GR, Reingold SC. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler. 1999;5(4):244-250.
12. Rocca MA, Colombo B, Falini A, Ghezzi A, Martinelli V, Scotti G, Comi G, Filippi M. Cortical adaptation in patients with MS: a cross-sectional functional MRI study of disease phenotypes. Lancet Neurol. 2005;4(10):618-626.
Figures