4644
Longitudinal 7T MRI and MRS in a sheep model of Tay-Sachs disease and the effect of AAV gene therapy.1Auburn University, Auburn, AL, United States, 2MR R&D, Seimens Healthcare, Malvern, PA, United States, 3University of Massachusetts, Worcester, MA, United States
Synopsis
Tay-Sachs Disease (TSD) is a fatal neurodegenerative disorder of children and the sheep model of TSD is a powerful tool to study the disease and evaluate novel therapies. One such therapy, adeno associated viral (AAV) gene therapy has resulted in a 2-fold increase in lifespan and biomarkers are needed for clinical trials. 7T MRI shows white and gray matter alterations and MR spectroscopic abnormalities that worsen with TSD disease progression. At humane endpoint the AAV treated sheep has normalization of gray and white matter intensities, but cortical atrophy and MRS alterations persist. 7T MRI and MRS reflect TSD disease severity.
Introduction: Tay-Sachs Disease (TSD) is a fatal neurodegenerative disorder of children caused by a deficiency in the heterodimeric enzyme hexosaminidase A (Hex). Ovine TSD is the only experimentally relevant animal model of TSD and is a powerful tool to study disease as well as test novel therapies like adeno associated viral (AAV) gene therapy.
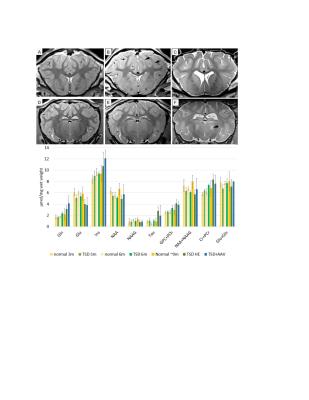
Methods: One of the therapeutic challenges of Tay-Sachs and Sandhoff disease is the requirement for simultaneous delivery of the genes for both Hex subunits (α and β). We developed a dual gene construct that results in simultaneous expression of both subunits from a single vector. TSD sheep were treated at 2-3 months of age by thalamic and lateral ventricle injection of 6×1012 v.g. of an AAVrh8 vector encoding the α and β subunits. MRI and MRS were performed at 3, 6 and 9 months in untreated TSD sheep and at humane endpoint (HE) in AAV treated sheep. Anatomical transverse 2D axial T2 turbo spin echo (TSE) images were acquired with TR/TE of 4000/12ms and a resolution of (0.5x0.5x1) mm. Single voxel spectroscopy (SVS) was then acquired using Stimulated Echo Acquisition Time (STEAM) with Variable Pulse power and Optimized Relaxation Delays (VAPOR) water suppression, TE/TR = 4.6/10000 ms and 32 averages. In all animals a (10x10x17) mm3 voxel was placed in the striatothalamic region that was well defined on the high resolution anatomical images. MRI data were analyzed with EFilm 3.2 software (Merge Healthcare, Chicago). MRS data were processed with LC model and internal water scaling (http://www.s-provencher.com/pages/lcmodel.shtml).
Results: AAV gene therapy in the TSD sheep (TSD+AAV) resulted in an extension of lifespan to approximately twice that of TSD sheep (9.1±1.1month survival), with the oldest sheep still ongoing at 19.2 months. The TSD sheep (B,C) at HE has intensity inversion at the gray-white matter interface (arrow) compared to the normal sheep (A,D). After AAV gene therapy this MRI abnormality is normalized, however cortical atrophy persists (E, F). MRS (bottom) of the TSD sheep thalamus showed trending increases in glutamine (Gln), myo-iniositol (Ins), taurine (Tau), glycerophosphocholine + phosphocholine (GPC+PCh) and creatine + phosphocreatine (Cr+PCr). Trending decreases were noted in glutamate (Glu) and N-acetylaspartate (NAA). At humane endpoint AAV treated sheep had MRS metabolite alterations similar to the untreated TSD sheep.
Conclusions: This success has prompted planning of human clinical trials, but objective measures to track disease are needed prior to onset. In the TSD sheep MRI and MRS suggest increasing neurodegeneration in the TSD sheep with time. Alterations in the glutaminergic system, gliosis (Ins) neuroaxonal health (NAA), myelination (GPC+PCh) are in line with changes in other neurodegenerative disorders. Increased taurine likely represents taurine conjugated GM2 a toxic metabolite reported in Tay-Sachs 1. The AAV treated sheep at humane endpoint revealed normalization of gray and white matter intensities, however cortical atrophy and MRS metabolite alterations persisted. Continuation of these studies will evaluate level of correction of MRI and MRS at intermediate time points in AAV treated TSD sheep.
Acknowledgements
BioMarin Pharmaceutical
National Tay-Sachs and Allied Diseases Foundation
Scott-Ritchey Research Center, Auburn University, AL, USA
References
References: 1. J Biol Chem. 2003 Sep 12;278(37):35286-91Figures