4641
Abnormal Thalamic Metabolism in End-Stage Renal Disease Patients with Restless Legs Syndrome1Medical Imaging, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, People's Republic of China, 2Imaging Department, NO.215 Hospital of Shaanxi Nuclear Industry, Xianyang, People's Republic of China
Synopsis
Restless legs syndrome (RLS) was very common among ESRD patients. However, the physiopathologic mechanisms of RLS in ESRD patients were largely unclear. Here we analyzed the thalamic metabolites using 1H MRS and the correlation between sleep/RLS score and metabolites, compared with corresponding normal controls. The results showed that the Cho/Cr ratio increased and NAA/Cr ratio decreased in thalamus of ESRD patients. The correlation between Cho/Cr ratio and RLS score was significant. It indicated that the sleep disturbance in ESRD patients with RLS might be associated with the abnormal thalamocortical activation of human sleep regulation system.
Introduction
As the chronic kidney disease (CKD) progressed to end-stage renal disease (ESRD) which the estimated glomerular filtration rate (eGFR) was less than 15 ml/min/1.73 m2, there was the need for renal replacement therapy by hemodialysis, peritoneal dialysis, and transplantation1. Restless legs syndrome (RLS) was a sensorimotor neurological disorder characterized by an urge to move the limbs and unpleasant sensations, which was much higher than common population in the ESRD patients with the prevalence ranged from 12% to 57% (average 30%)2. However, the physiopathologic mechanisms of RLS in ESRD patients were largely unclear. More and more researches have been demonstrated that the thalamus also play a crucial role in human sleep-related activity3. In this study, we choose proton magnetic resonance spectroscopy (MRS) to detect the metabolic changes of thalamus, focusing on exploring the relationships between sleep disturbances and thalamic metabolism in hemodialysis patients with RLS.Methods
Twenty-three hemodialysis patients with RLS (12 men, 11 women; aged 21-50 years, mean age 34.26±8.55years) were included in our study based on the diagnostic criteria of International Restless Legs Syndrome Study Group (IRLSSG)7. Twenty-two age-matched healthy volunteers (12 men, 10 women; aged 20-48years, mean age 33.48±10.37 years) were recruited as control group. All of the subjects underwent Pittsburgh Sleep Quality Index (PSQI) scale to evaluate the sleep conditions. Brain scanning was performed on a GE Signa VH/i wholebody 3.0-T system. Axial T2-weighted fast spin-echo sequences were obtained for image-guided localization of voxels of interest (VOIs) for MRS. Multi-voxel 1H-MRS spectra with long echo time (TE = 144 ms) was acquired using point-resolved spectroscopy sequence (PRESS) in the bilateral thalami. Cerebral metabolites including N-acetylaspartate (NAA), creatine (Cr) and choline (Cho) were calculated using a GE ADW4.4 workstation. Metabolite ratios using Cr as an internal standard for NAA, Cho were calculated automatically at the central area of bilateral thalami. For statistical analysis, metabolite ratios were compared between patients and controls using an unpaired Student’s t-test. Correlations between each metabolite ratio and RLS score were conducted using Pearson’s correlation coefficient.Results
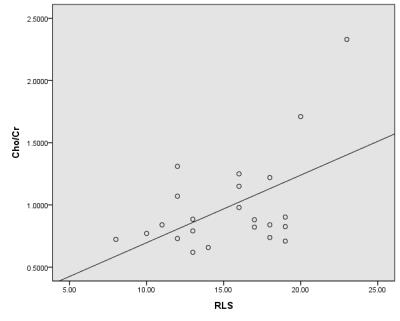
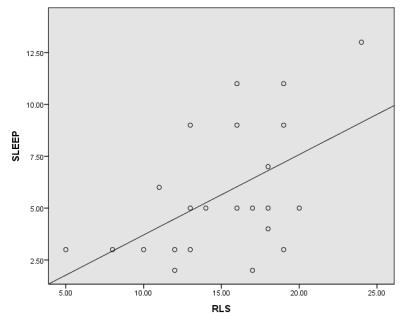
ESRD patients displayed significant elevations of the Cho/Cr ratio in thalamus compared with control subjects (0.99±0.38 versus 0.77±0.21, P<0.05), in addition to significantly reduced NAA/Cr ratio in thalamus (1.70±0.41 versus 1.97±0.42, P<0.05). Significant positive correlations were found between the Cho/Cr ratio and RLS score (r=0.52, p<0.01) (Fig.1). The RLS score was correlated with PSQI score significantly (r=0.53, P<0.01) (Fig.2).Discussion
Using 1H-MRS, we detected a significant reduction in the neuronal marker NAA and elevation in the neuronal injury Cho of thalamus in the ESRD patients with RLS. Thalamus is a central part in human ascending reticular activating system (ARAS) which is very important for sleep-related activity4. Our finding indicates that the mechanism producing sleep disturbance in uremic RLS patients may involve abnormal thalamocortical activation. For ESRD patients, many factors may be responsible for the metabolic disturbance. The accumulation of uremia toxin such as creatinine and urea may affect the osmotic stress of cerebral cell5. Anemia is another important factor for the brain metabolic changes6. The long-term anemia secondary to ESRD could decrease the oxygen supply of brain, resulting in cerebral hypoxia, which was one of main reasons for brain injury7. Besides, some researches also indicate that parathormone (PTH) and fluid overload may be related to the abnormal metabolism in ESRD patients8,9.Conclusion
The metabolites of thalamus in ESRD patients with RLS are abnormal, which may lead to the changes of ARAS and finally cause the sleep disturbance. Some suspefactors are suspected to be associated with the abnormal metabolism of thalamus, but still it needs further more studies.Acknowledgements
The authors report no conflicts of interest.References
1. Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol 2007;18(10):2644-8.
2. Giannaki CD, Hadjigeorgiou GM, Karatzaferi C, Pantzaris MC, Stefanidis I, Sakkas GK. Epidemiology, impact, and treatment options of restless legs syndrome in end-stage renal disease patients: an evidence-based review. Kidney Int 2014;85(6):1275-82.
3.Coulon P, Budde T, Pape HC. The sleep relay--the role of the thalamus in central and decentral sleep regulation. Pflugers Arch 2012;463(1):53-71.
4. Krone L, Frase L, Piosczyk H, Selhausen P, Zittel S, Jahn F, Kuhn M, et al. Top-down control of arousal and sleep: Fundamentals and clinical implications. Sleep Med Rev. (2016) 1-8.
5. Walker S, Fine A, Kryger MH. Sleep complaints are common in a dialysis unit. Am J Kidney Dis 1995;26(5):751-6.
6.Roger SD, Harris DC, Stewart JH. Possible relation between restless legs and anaemia in renal dialysis patients. Lancet 1991;337(8756):1551.
7.Kuwabara Y, Sasaki M, Hirakata H, Koga H, Nakagawa M, Chen T, et al. Cerebral blood flow and vasodilatory capacity in anemia secondary to chronic renal failure. Kidney Int 2002;61(2):564-9.
8. Sabbatini M, Minale B, Crispo A, Pisani A, Ragosta A, Esposito R, et al. Insomnia in maintenance haemodialysis patients. Nephrol Dial Transplant 2002;17(5):852-6.
9. Elias RM, Chan CT, Bradley TD. Altered sleep structure in patients with end-stage renal disease. Sleep Med 2016;20:67-71.