4640
MRS-based classification of Spinocerebellar Ataxias 1, 2, 3, and 6 at 7T: A distance-weighted discrimination approach1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 2Division of Biostatistics, University of Minnesota, Minneapolis, MN, United States, 3University of Oxford, Oxford, United Kingdom, 4Department of Neurology, University of Chicago, Chicago, IL, 5Department of Neurology, University of Minnesota, Minneapolis, MN
Synopsis
We investigated the sensitivity of ultra-high field MRS to distinguish 4 hereditary neurodegenerative diseases with substantial overlap in clinical presentation and conventional MRI. We carried out pairwise classifications of spinocerebellar
Introduction
Spinocerebellar ataxias (SCAs) are hereditary movement disorders that cause neurodegeneration in the cerebellum and, in many cases, the brainstem. The most common SCAs worldwide, SCA1, SCA2, SCA3 and SCA6 are polyglutamine diseases. They show substantial overlap in clinical presentation and conventional MRI.1 Identifying robust imaging markers that can differentiate between SCA types can guide genetic testing and improve understanding of differences in the disease processes. Neurochemical profiles acquired from the cerebellum and brainstem regions using high field magnetic resonance spectroscopy (MRS) showed differences in specific metabolites across SCA1, SCA2 and SCA6 in a pilot study, however no individual metabolite level was able to accurately classify different SCA types2. Here we investigated the specificity of MRS markers in a large cohort with SCA, including all 4 common SCAs, and took advantage of sensitivity gains at ultrahigh field. We utilized a distance weighted discrimination3 (DWD) approach that combines metabolic profiles from several anatomic regions and thereby may provide a robust means by which to classify these clinically and pathologically similar SCA types.Methods
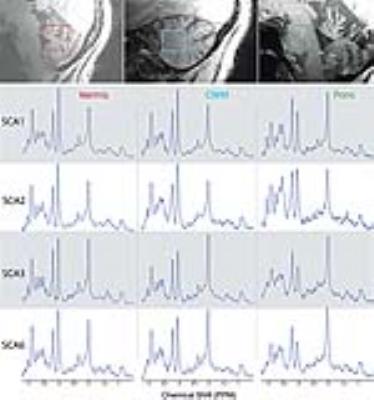
Sixty-eight genetically-confirmed subjects with early-moderate stage SCA {SCA1 (n=18, age(SD)=48.8(11.5) years), SCA2 (n=16, age(SD)=44.3(11.1)), SCA3 (n=15, age(SD)=51.5(12.5)), SCA6 (n=19, age(SD)=61.7(9.5))} were studied cross-sectionally. 7T MR spectra were acquired from the cerebellar vermis (25x10x25mm3), cerebellar white matter (CWM) (17x17x17mm3), and pons (16x16x16mm3) (figure 1) using a short-echo, semi-LASER sequence4 (TR/TE=5000/26 ms, 64 transients) and a 16 channel transceiver that permitted the B1 transmit field to be shimmed for each region. Single-shot spectra were eddy-current, phase and frequency corrected prior to summation and fitting with LCModel5, using water scaling, as described previously6. Metabolites with mean Cramer-Rao lower bounds (CRLB) ≤ 20% in any of the SCA cohorts were included in the DWD analysis. Subject age and gender were also included in the DWD input vector. DWD is a machine learning algorithm that finds an optimized hyperplane separating two or more groups based on maximizing the distance between the nearest points on each side of the hyperplane to the hyperplane. Mis-classification was quantified using leave-one-out cross-validation. The Scale for the Assessment and Rating of Ataxia (SARA)7 was used as a measure of disease burden, and yields a composite ataxia score in the range of 0 (no ataxia)—40 (most severe ataxia). The recruitment target score ranged from 0 (no symptoms) to 15 (early disease stage).Results and Discussion
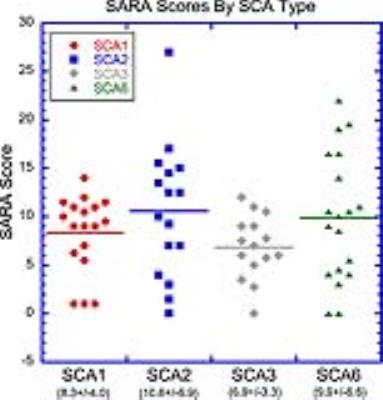
MR spectral quality was generally very good across subjects (figure 1). The DWD results include a rank of the input components driving each of the SCA classifications, of which, the five most important for each pair of comparisons are listed in table 1. Classifications were driven strongly by metabolite levels in the CWM and the pons. Gender was not a major discriminator for any of the pairwise comparisons and age was an important discriminator only between SCAs 2 and 6. This is not unexpected since SCA2 symptom onset is typically the earliest of the SCAs studied here, while SCA6 symptom onset is significantly delayed compared to SCAs 1-3.8 SARA scores indicated varied degrees of SCA involvement across subjects, but were not significantly different among groups (figure 2).
DWD classification of SCA6 between each of SCAs 1-3 was very good as shown by the binary classification plots in figure 3. These results are in agreement with differences in disease pathology of SCAs 1-3 to those of SCA6, specifically the lack of involvement of the pons in SCA6.1 Additionally, figure 3 indicates that our approach to SCA classification was moderately successful in classifying pathologically similar SCA2 and SCA3. A full summary of misclassifications and their rates for each of the binary DWD SCA comparisons is provided in table 2. Classifications of SCA1 from SCAs 2 and 3 were the most challenging with our current model.
Conclusion
Here we show that 7T MRS and DWD analysis results in highly reliable classification of subjects with SCA6 from those with SCAs 1-3. Additionally, our approach is moderately successful in the classification between SCAs 2 and 3. Increased subject numbers or higher dimensionality including MRS of other implicated regions or measures such as regional fractional anisotropies and/or structural volumes may afford further discrimination of hard-to-differentiate SCA types.Acknowledgements
This work was supported by NIH grants R01 NS070815, R01 NS080816, P41 EB015894 and P30 NS076408.References
[1] Taroni F and DiDonato S. Pathways to motor incoordination: the inherited ataxias. Nat Rev Neurosci 2004; 5(8):641-655.
[2] Oz G, Itis I, Hutter D, et al. Distinct Neurochemical Profiles of Spinocerebellar Ataxias 1, 2, 6, and Cerebellar Multiple System Atrophy. Cerebellum 2011; 10:208–217.
[3] Marron JS, Todd MJ and Ahn J. Distance-Weighted Discrimination. JASA 2007;102(480):1267-1271.
[4] Oz G and Tkac I. Short-echo, single-shot, full-intensity proton magnetic resonance spectroscopy for neurochemical profiling at 4 T: validation in the cerebellum and brainstem. Magn Reson Med 2011; 65(4):901-910.
[5] Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993; 30(6):672–679.
[6] Deelchand DK, Adanyeguh IM, Emir UE, et al. Two-Site Reproducibility of Cerebellar and Brainstem Neurochemical Profiles With Short-Echo, Single-Voxel MRS at 3T. Magn Reson Med 2015; 73(5):1718-1725.
[7] Schmitz-Hübsch T, du Montcel ST, Baliko L et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 2006; 66(11):1717–20.
[8] Schmitz-Hübsch T, Coudert M, Bauer P et al. Spinocerebellar ataxia types 1, 2, 3, and 6 Disease severity and nonataxia symptoms. Neurology 2008; 71(13):982-989.
Figures