4638
Individual mapping of neuronal damage in early relapsing-remitting MS using [11C]Flumazenil PET1Institut du Cerveau et de la Moelle Epinière/CNRS UMR 7225/INSERM 1127/UPMC UM75, Paris, France, Paris, France, 2CEA, DRF, I2BM, Neurospin, UNIRS, Paris, France, 3rtement of medecine, Surgery and Neuroscience, University of Siena, Siena, Italy, Italy, 4CEA, DRF, I2BM, Neurospin, UNIACT, Paris, France
Synopsis
We explored the neuronal component of grey matter damage in the earliest phase of multiple sclerosis (MS) with [11C]Flumazenil positron emission tomography (PET). Using a novel post-processing approach based on the generation of individual maps of neuronal pathology, we found a significant neuronal damage in the cortical lesions of patients with MS which preceded the occurrence of cortical atrophy, and correlated with white matter lesion load. These results suggest that cortical demyelination, together with retrograde degeneration of transected axons within white matter lesions, are among the key pathogenic contributors to neuronal cortical damage at the earliest stage of the disease.
Introduction
Grey matter (GM) damage has been identified as one of the major pathological mechanisms responsible for disability progression in multiple sclerosis (MS)1. The pathological substrate underlying GM damage is complex, and includes cortical demyelination, inflammation, synapse and dendrite loss, and neuronal cell death. One of the key unanswered questions in the pathophysiology of MS is the role played by the neuronal component of GM damage in the earliest stage of the disease. We have recently proposed positron emission tomography (PET) with [11C]Flumazenil (FMZ), an antagonist of the central benzodiazepine site located within the GABAA receptor, as a promising biomarker to specifically identify and measure the neuronal component of GM pathology in-vivo in patients with MS2. The aim of this study is to explore neuronal damage in the earliest phase of relapsing-remitting MS employing [11C]FMZ PET to generate individual cortical maps of neuronal pathology, and to investigate the relationship between the neuronal component of GM damage and grey and white matter lesions.Methods
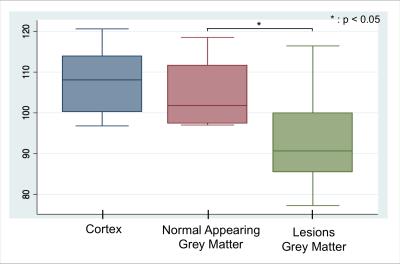
Eleven patients within 2 years of the diagnosis of relapsing-remitting MS, and 6 age- and gender- matched healthy controls (HC) were included in this study and underwent a clinical and neuropsychological assessment as well as a full imaging protocol on a 3T MRI scanner and a PET scan with [11C]FMZ. [11C]-FMZ cortical binding was estimated using the partial saturation protocol, which provides parametric maps of the absolute quantification of Bmax3 (Fig 1a), that reflects the concentration of GABAA receptor. Cortical maps of significant differences in the tracer binding between patients and HC, adjusted for age, gender and specific radioactivity of injected doses, were therefore employed to establish a threshold of significantly decreased tracer binding in each voxel, reflecting the presence of selective neuronal damage (yielding a threshold of 30%). This allowed to generate individual maps of neuronal damage (Fig 1b), which were used to calculate the percentage of abnormal voxels over the whole cortical volume in each subject. Differences in GM normalized volume between patients and HC were assessed using SIENAX4. Significant differences in mean [11C]FMZ binding between cortical lesions and normal-appearing cortex in patients, and the whole GM in HC, were estimated using linear regressions adjusted for age and gender. In patients, the Spearman’s rank correlation coefficient was used to explore the correlation between the percentage of pathological voxels and white matter lesion load.Results
While
we found no significant difference in normalized GM volume nor in
mean Bmax in normal appearing grey matter between patients and HC, a
significant decrease in Bmax was demonstrated between GM lesions in
patients and cortical GM in HC. (Patients: 93.9 ± 11.5 pmol/ml ; Controls: 108.0 ±8.9 pmol/ml) (p < 0.05) (Fig 2).
The degree of neuronal damage was heterogeneous across patients,
with the percentage of abnormal voxels ranging from 5% to 20% of
total cortical volume. A significant correlation was found between
the percentage of pathological voxels and white matter lesion load
(p=0.03, rho=0.64).Discussion & Conclusion
[11C]FMZ PET allowed to measure in-vivo the neuronal component of GM damage in patients with MS, which is detectable mainly in cortical lesions since the earliest stage of the disease, before the onset of cortical atrophy. Through the development of a novel post-processing technique, we were able to generate individual maps of cortical neuronal damage, which demonstrated heterogeneous levels of pathological changes across the patient cohort. The decrease in Bmax was mostly localized within cortical lesions, which suggests that cortical demyelination might play a key role in determining neuronal damage at the earliest stage of the disease. The significant correlation we found between the percentage of cortical voxels characterized by a decreased [11C]FMZ binding and white matter lesion load, indicates that the Wallerian degeneration of transected axons within white matter lesions could be another critical contributor to the development of a secondary neuronal cortical damage5. The relative contribution of these two pathogenic components to the onset and the evolution of neuronal damage in early MS is currently being investigated through the tractography-based reconstruction of white matter tracts combined with the [11C]FMZ PET investigation of neuronal damage in connected cortical targets.Acknowledgements
EP was funded by the IUIS doctoral contract.
BB was supported by the ARSEP post-doctoral research fellowship.
TS, LF, DLG, GB and MB have nothing to disclose.
BS received honoraria from Biogen, Teva, Novartis, Genzyme, and research support from Genzyme and Merck-Serono. Research support: Programme National de Recherche Clinique (PHRC), APHP-DRCD (Assistance Publique des Hôpitaux de Paris), Investissements d’avenir” ANR-10-IAIHU-06 grant, GMSI-Merck Serono 2014
References
1. Calabrese, M., et al (2007). Cortical atrophy is relevant in MS at clinical onset. Journal of neurology, 254(9), 1212-1220.
2. Freeman, L., et al (2015). The neuronal component of GM damage in MS : A 11C-FMZ PET study. Annals of neurology, 78(4), 554-567.
3. Delforge, J., et al (1995). Quantification of benzodiazepine receptors in human brain using PET : 11C-FMZ and a single experiment protocol. Journal of Cerebral Blood Flow & Metabolism, 15(2), 284-300.
4. S.M. Smith, S.M., et al (2004). Accurate, robust and automated longitudinal and cross-sectional brain change analysis. NeuroImage, 17(1):479-489.
5. Bodini, B., et al (2016). White and gray matter damage in primary
progressive MS The chicken or the egg?. Neurology, 86(2), 170-176.
Figures
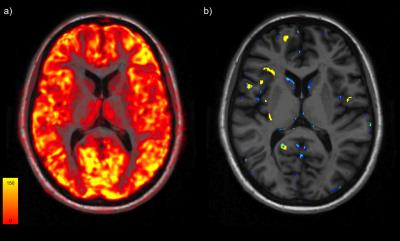
Fig 1 : Individual mapping of neuronal damage of a early RR MS patient. a) Bmax map quantified with the the partial saturation protocole onto anatomical sequence.b) Individual map of neuronal damage (blue) and cortical lesion mask (yellow).