4635
Sleep-Disordered Breathing Severity in Elderly is Associated with Decreased GABA in the Dorsolateral Prefrontal Cortex: A J-edited 1H MRS and Polysomnography Study1Laboratory of Neuroendocrinology, The Rockefeller University, New York, NY, United States, 2Radiology, Weill Cornell Medicine, 3Laboratory of Neuroendocrinology, The Rockefeller University, 4Medicine, Neurology and Neuroscience, Weill Cornell Medicine, 5Radiology, Weill Cornell Medicine, New York, NY, United States
Synopsis
Sleep-disordered breathing (SDB) is a disorder characterized by repeated episodes of hypopnea and apnea during sleep that lead to sleep fragmentation and intermittent hypoxia. A leading model of model of SDB that posits GABAergic and glutamatergic dysregulations that lead to hyperexcitability and neuronal damage, which this study aimed to investigate using J-edited MRS and polysomnography. The main results were a robust DLPFC GABA decrease and associations between GABA and hypoxia as well as disease severity. The state of hyperexcitability observed in SDB is interpreted as likely the result of disinhibition (GABA) that might lead to excitotoxicity and neuronal damage.
INTRODUCTION
Sleep-disordered breathing (SDB), prevalent in the elderly population, is characterized by repeated episodes of hypopnea and apnea during sleep that lead to sleep fragmentation and intermittent hypoxia1 – events that are believed to contribute to cognitive decline in individuals affected with SDB2-4. With mounting evidence suggesting that SDB-associated hypoxia can lead to hyperexcitability and neuronal damage5-7, a model of the disorder has emerged that posits GABAergic and glutamatergic dysregulations. Using J-edited 1H MRS, this study sought to test the hypothesis of GABA and/or glutamatergic compounds (“Glx”) are altered in the dorsolateral prefrontal cortex (DLPFC) and the hippocampus of elderly individuals with SDB, by determining both the magnitude and direction of these alterations relative to age-matched healthy volunteer (HV) subjects. Secondary analyses were conducted to assess whether there exist correlations between neurotransmitter and oxygenation levels that might associate hypoxia with neural excitability in SDBMETHODS
Subjects: For this study 19 elderly subjects with moderate-to-severe SDB, as assessed with the Apnea-Hypopnea Index (AHI)8, and 14 age-matched healthy subjects were recruited through referrals from the Center for Sleep Medicine at New York Presbyterian/Weill Cornell Medical Center. The study’s main assessments consisted of clinical evaluations, polysomnography and 1H MRS scans.
Brain GABA and Glx Measurements In Vivo by J-edited 1H MRS: In vivo edited GABA and Glx spectra were recorded in 15 min on a 3.0 T GE MR system from a 2.0 x 2.0 x 4.5-cm3 voxel in the DLPFC (Figure 1) and a 1.5 x 2.0 x 4.5-cm3 in the hippocampus (Figure 2), using J-edited MRS and an 8-channel phased-array head coil, with TE/TR 68/1500 ms and 290 interleaved excitations (580 total). Levels of GABA and Glx in each voxel of interest were derived by frequency-domain fitting (Figures 1 & 2) as peak area ratios relative to the unsuppressed intravoxel water (W) signal.
Polysomnography: Standard nocturnal polysomnography (NPSG) was performed using a Grass® Comet XL system with an integrated Nonin® oximetry to derive the data necessary for estimating the two key SDB outcome measures for this study: (a) the Apnea-Hypopnea Index (AHI)8 as a measure of SDB severity, and minimal oxygen (O2) saturation as a measure of oxygenation and hypoxia. General linear models were used to compare GABA/W and Glx/W between SDB and HV subjects. Pearson correlations were used to test for associations between neurotransmitter levels and AHI and oxygenation.
RESULTS
In subjects with SDB, levels of DLPFC GABA, but not Glx, were significantly lower than in control subjects (p < 0.0002) (Figure 3). In addition, there was a negative association between DPLFC GABA levels, but not Glx, and SDB severity or AHI (r=-0.69, p<0.0001) (Figure 4A), and a positive association between DLPFC GABA levels, but not Glx, and minimal O2 saturation (r=0.68, p=0.0005) (Figure 4B). By contrast, no group differences or associations were found between AHI or oxygen saturation levels GABA, Glx or any other metabolite in the hippocampus.
DISCUSSION AND CONCLUSION
To our knowledge, the present study is the first to measure and report neurometabolic features in elderly subjects with SDB, finding robustly decreased levels of GABA in the DLPFC compared to age-matched healthy control subjects (Figure 3). In addition, GABA levels in this study correlated negatively with the severity of sleep apnea as assessed with AHI (Figure 4A), and positively correlated with minimal O2 saturation (Figure 4A), both only in the DLPFC, indicating that high disease severity and low oxygenation – both of which are non-salutary – are associated with decreased GABAergic tone. The state of hyperexcitability that has been reported in preclinical models of hypoxia5-7, therefore, may result from decreased inhibitory neurotransmission tone. Excitotoxicity that might result from the observed robust GABA decrease and associated disinhibition of glutamatergic excitation could conceivably lead, in the long-term, to neuronal damage and cognitive deficits in SDB.
In conclusion, this study has reported, for the first time, human brain data documenting a robust DLPFC GABA deficit in subjects with SDB. Along with the totality of available preclinical and clinical data, this GABAergic deficit has been interpreted as supporting a pathophysiological model of the disorder in which sleep disordered breathing, with intermittent hypoxia, leads to the observed GABA decrease, which culminates in neural hyperexcitability – likely by disinhibiting pyramidal neurons – leading to excitotoxicity, neuronal damage, and, ultimately, to cognitive decline in SDB.
Acknowledgements
Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. 1991. Sleep-disordered breathing in community-dwelling elderly. Sleep 14: 486-95.
Spira AP, Blackwell T, Stone KL, Redline S, Cauley JA, et al. 2008. Sleep-disordered breathing and cognition in older women. J Am Geriatr Soc 56: 45-50.
Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, et al. 2011. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. Jama 306: 613-9.
Zimmerman ME, Aloia MS. 2012. Sleep-disordered breathing and cognition in older adults. Curr Neurol Neurosci Rep 12: 537-46.
Schiff SJ, Somjen GG. 1985. Hyperexcitability following moderate hypoxia in hippocampal tissue slices. Brain research 337: 337-40.
Turovsky EA, Turovskaya MV, Kononov AV, Zinchenko VP. 2013. Short-term episodes of hypoxia induce posthypoxic hyperexcitability and selective death of GABAergic hippocampal neurons. Exp Neurol 250: 1-7.
Yuan L, Wu J, Liu J, Li G, Liang D. 2015. Intermittent Hypoxia-Induced Parvalbumin-Immunoreactive Interneurons Loss and Neurobehavioral Impairment is Mediated by NADPH-Oxidase-2. Neurochem Res 40: 1232-42.
Ruehland WR, Rochford PD, O'Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009 Feb;32(2):150-7
References
Acknowledgments: This work was supported by the Iris and Jumming Le Foundation, the DANA Foundation, the Rockefeller University Women & Science Initiative and NIH grant 1 K76AG054772 to A.C.P.; NIH grant 1 R01MH075895 to D.C.S.; and partial support by grant # UL1TR001866 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program.Figures
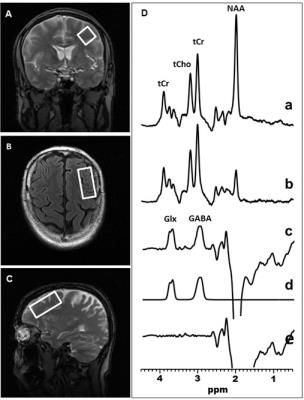
Figure 1: Illustration of brain left DLPFC GABA and Glx detection by J-edited difference 1H MRS, followed by frequency-domain fitting of the difference spectrum (c) to obtain GABA and Glx peak areas that yield their levels.
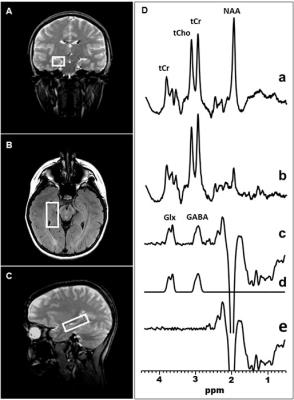
Figure 2: Illustration of brain right hippocampal GABA and Glx detection by J-edited difference 1H MRS, followed by frequency-domain fitting of the difference spectrum (c) to obtain GABA and Glx peak areas that yield their levels.
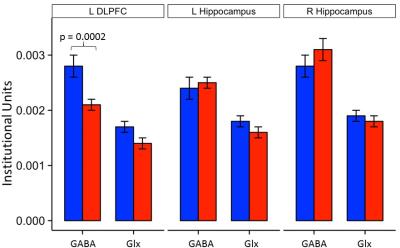
Figure 3: Left DLPFC and hippocampal GABA and Glx levels in patients with sleep disordered breathing (red) and healthy controls (blue).
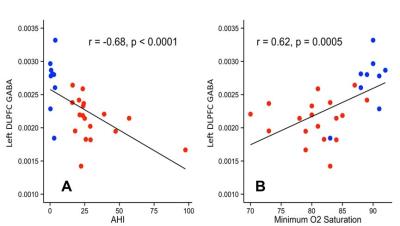
Figure 4: Linear correlation plots between [A] Apnea Hypopnea Index (AHI) left DLPFC GABA, and [B] between minimum O2 saturation and left DLPFC GABA in patients with sleep disordered breathing (red) and controls (blue).