4634
Regional GABA concentrations modulate inter-network resting-state functional connectivity1McLean Hospital, Belmont, MA, United States, 2National Institute on Drug Abuse, Baltimore, MD, United States
Synopsis
The resting-state functional connectivity (rsFC) and task evoked fMRI, involving the default mode network (DMN) and control network (CN) was performed, with GABA, glutamate and glutamine measured at MPFC and DLPFC, in order to explore the underlying molecular mechanism of the DMN-CN interaction. We found that MPFC GABA concentrations significantly modulate DMN deactivation during a working memory task, and resting state anti-correlation between DMN and CN, while DLPFC GABA correlations modulate DMN-CN anti-correlation in the opposite direction. These findings suggest that MPFC and DLPFC GABA make a major but differential impact on task related activation and inter-network rsFC. The neurochemical characteristics of DMN and CN may provide novel insights into abnormal network activity in neuropsychiatric diseases and provide opportunities for novel interventions.
Introduction
During externally-oriented attention-demanding tasks, the default mode network (DMN) is deactivated while task-related other networks including the frontoparietal control network (CN) are activated in the human brain (1). Compromised DMN deactivation and CN activation as well as decreased resting state anti-correlation between DMN and CN have been reported in various neuropsychiatric disorders (2,3). The underlying neural and molecular mechanism for these abnormalities, however, is still elusive. In the current study, we measured resting state functional connectivity (FC) and task-related brain activity with tasks intended to activate the DMN and CN, as well as the concentrations of GABA, glutamate (Glu) and glutamine (Gln) at critical nodes (medial prefrontal cortex (MPFC) and dorsolateral prefrontal cortex (DLPFC)) within the DMN and CN from a group of healthy individuals. We hypothesized that glutamatergic and GABAergic measures would predict DMN-CN anti-correlation measures at rest and during task-related activation.Methods
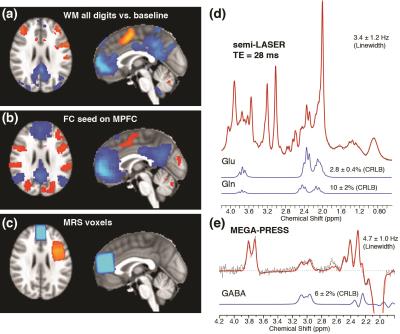
19 healthy subjects (6 males, mean ± SD of age: 25.8 ± 5.0 years) underwent the following MR scans at a Siemens 3T Trio scanner: a 5-min high-resolution anatomical scan, 6-min resting fMRI, followed by 14-min MRS and 6-min working memory (WM) fMRI. MEGA-PRESS (TE/TR = 68/3000 ms; total 192 averages) (4,5) and semi-LASER (6,7) (TE/TR = 28/3000 ms; 64 averages) sequences were acquired at MPFC (30x20x30 mm3) and DLPFC (25x25x35 mm3) to measure GABA and Gln/Glu respectively. MRS data were processed in FID-A (8) and quantified using LCModel. Metabolite concentrations were referenced to water with partial volume corrections.
Resting and WM task fMRI data with whole-brain coverage were collected using a single-shot GE-EPI sequence (TR/TE = 2500/30 ms; slice thickness/gap = 4/0 mm; in-plane resolution = 3.44×3.44 mm2). All subjects performed a block-design Steinberg task (2 blocks each for 1-digit, 3-digit and 5-digit, respectively). Task fMRI data were analyzed in FSL5.0, and the resting-state fMRI were analyzed in DPARSFA (9) with standard steps. General linear modeling was used to assess task-induced brain activity. The MPFC/DLPFC regions showing WM task-induced deactivation/activation were defined as the seed regions in the functional connectivity analysis. Relationships between neurotransmitter concentrations and DMN deactivation, as well as functional connectivity between DMN and CN were examined.
Results and discussions
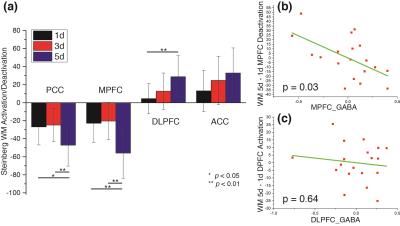
In WM fMRI analysis, different cognitive loads (1d, 3d and 5d) were examined separately (Fig. 2a). A multiple regression analysis was performed to regress DLPFC/MPFC activation/deactivation of the contrast of different loads (5d-1d, 3d-1d, 5d-3d respectively) on age, gender, GM% of MRS voxel, GABA, Glu, and Gln/Glu ratio of the same region. It was observed that MPFC GABA was significantly correlated with MPFC deactivation in the regression models under the 5d-1d (Fig. 2b) and 5d-3d conditions but not 3d-1d. This observation is consistent with a recent report in the PCC region (10). On the other hand, we found no significant correlation in the regression models for the DLPFC activation for any metabolites measured within the MPFC or DLPFC (Fig. 2C).
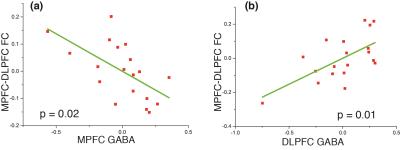
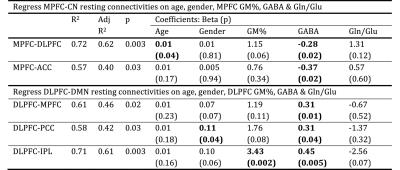
We also carried out a multiple regression analysis to regress inter-network resting-state functional connectivity (rsFC) against age, gender, gray matter percentage (GM%) of MRS voxel, GABA, Glu and Gln/Glu ratio of the seed region. The results are presented in Table 1 and represent partial regression plots of MPFC-DLPFC rsFC with MPFC/DLPFC GABA are shown in Fig. 3. Among the independent variables, GABA concentration in both the MPFC and the DLPFC is the only measure consistently associated with between-network connectivity. The GABA concentrations within MPFC and DLPFC, however, modulate inter-network rsFC in opposite directions: higher GABA concentration in MPFC predicts stronger anti-correlation between DMN and CN (Fig. 3a), while higher GABA concentration in DLPFC predicts weaker anti-correlation (Fig.3b). The Gln/Glu ratio shows trends in the opposite directions compared to GABA in all models but none of these are significant. Glu itself gives a slope close to zero in the partial regressions. The absence of an impact of Glu and Gln/Glu on inter-network rsFC may be due to the dual metabolic/synaptic nature of the Glu signal quantified by 1H MRS.
Conclusion
We found that MPFC GABA concentrations significantly modulate DMN deactivation during a WM task, and the resting state anti-correlation between DMN and CN. On the other hand, DLPFC GABA concentrations do not predict task-related DLPFC activation but modulate resting state DMN-CN anti-correlation in the opposite direction. These findings suggest that MPFC and DLPFC GABA activity make important but differential impacts on task related activation and inter-network resting-state functional connectivity. The neurochemical characteristics of DMN and CN may provide novel insights into abnormal network activity in neuropsychiatric diseases and provide opportunities for novel interventions for these conditions.Acknowledgements
The authors thank Drs. Dinesh Deelchand (UMN), Gulin Oz (UMN), Malgorzata Marjanska (UMN), Edward J. Auerbach (UMN and Siemens), Susan Whitfield-Gabrieli (MIT) and Daphne Holt (MGH) for their assistance in the experiments and thoughtful discussions. This work was partially supported by grants from NARSAD (F.D.), MH094594 (D.O.), MH104449 (D.O.)References
1. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 2008;1124:1-38.
2. Chai XJ, Whitfield-Gabrieli S, Shinn AK, Gabrieli JD, Nieto Castanon A, McCarthy JM, Cohen BM, Ongur D. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2011;36(10):2009-2017.
3. Baker JT, Holmes AJ, Masters GA, Yeo BT, Krienen F, Buckner RL, Ongur D. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA psychiatry 2014;71(2):109-118.
4. Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed 1998;11(6):266-272.
5. Marjanska M, Lehericy S, Valabregue R, Popa T, Worbe Y, Russo M, Auerbach EJ, Grabli D, Bonnet C, Gallea C, Coudert M, Yahia-Cherif L, Vidailhet M, Meunier S. Brain dynamic neurochemical changes in dystonic patients: a magnetic resonance spectroscopy study. Mov Disord 2013;28(2):201-209.
6. Oz G, Tkac I. Short-echo, single-shot, full-intensity proton magnetic resonance spectroscopy for neurochemical profiling at 4 T: validation in the cerebellum and brainstem. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 2011;65(4):901-910.
7. Terpstra M, Cheong I, Lyu T, Deelchand DK, Emir UE, Bednarik P, Eberly LE, Oz G. Test-retest reproducibility of neurochemical profiles with short-echo, single-voxel MR spectroscopy at 3T and 7T. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 2015.
8. Simpson R, Devenyi GA, Jezzard P, Hennessy TJ, Near J. Advanced processing and simulation of MRS data using the FID appliance (FID-A)-An open source, MATLAB-based toolkit. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 2015.
9. Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB Toolbox for "Pipeline" Data Analysis of Resting-State fMRI. Front Syst Neurosci 2010;4:13.
10. Hu Y, Chen X, Gu H, Yang Y. Resting-state glutamate and GABA concentrations predict task-induced deactivation in the default mode network. The Journal of neuroscience : the official journal of the Society for Neuroscience 2013;33(47):18566-18573.
Figures