4630
Feasibility of 1H-MRS brain temperature map to detect hemodynamic abnormality in patients with unilateral chronic major cerebral artery steno-occlusive disease1Department of Neurosurgery, School of Medicine, Iwate Medical University, Morioka, Japan, 2Center for Information and Neural Networks (CiNet), NICT and Osaka University, Suita, Japan, 3Biofunctional Imaging, Immunology Frontier Reseach Center, Osaka University, Suita, Japan, 4Division of Ultra-High Field MRI, School of Medicine,Iwate Medical University, Morioka, Japan, 5Cyclotoron Reseach Center, School of Medicine, Iwate Medical University
Synopsis
Deep cerebral white matter (CWM) like the centrum semiovale is vulnerable to ischemic injury. Brain temperature (BT) was associated with cerebral hemodynamic abnormalities in patients with chronic ischemia. We investigated whether the BT distribution map by multi-voxel 1H-MRS were associated with the cerebral hemodynamic abnormalities assessed by positron emission tomography (PET) in the CWM region of patients with unilateral chronic major cerebral artery steno-occlusive disease. The BT map quantitatively and qualitatively correlated with PET images, especially oxygen extraction fraction. This may help to identify the patients at high risk for the stroke recurrence.
INTRODUCTION
Perfusion pressure in the deep cerebral white matter (CWM) region where the superficial perforators of the middle cerebral artery and the anterior cerebral artery feed blood can be relatively low, the CWM region thus may be more vulnerable than the cerebral cortex to ischemic injury1,2. By this reason, identification of the hemodynamic abnormalities in the CWM may help to prevent stroke recurrence in chronic ischemic patients. Brain temperature (BT) was associated with cerebral hemodynamic abnormalities in patients with acute stroke, chronic ischemia or CO poisoning3-5. In particular, multi-voxel 1H-MRS can show the BT distribution as a topography5, it thus may be able to help us to assess the hemodynamic abnormalities in the CWM region in patients with chronic ischemia. Here, we investigated whether the BT in the CWM region by multi-voxel 1H-MRS correlated with the cerebral hemodynamic abnormalities assessed by positron emission tomography (PET) in patients with unilateral chronic major cerebral artery steno-occlusive disease.METHODS
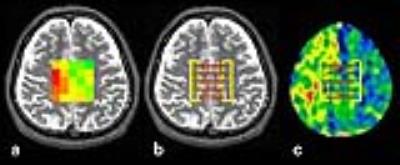
All MRI acquisitions were performed in 35 patients with unilateral middle cerebral or internal carotid artery steno-occlusive disease using a 3TMRI (SIGNA Excite HD; GE Healthcare, Milwaukee, WI) with a birdcage quadrature head coil and all patients underwent the MRI 1 month after the last ischemic event. For multi-voxel 1H-MRS, 5×5-voxel regions of interest (ROIs) were manually and symmetrically placed at the central semiovale on the T2-weighted (T2W) image, as locating the central row of voxels on the cerebral interhemispheric fissure (Figure 1). As the results, rows of voxels at left and right side edge of the ROIs approximately covered the CWM region in each cerebral hemisphere. Multi-voxel 1H-MRS acquisition with partial water decoupling was performed with the following parameters: voxel size, 10×10×15 [mm3]; TR/TE, 2,000/144 [ms]; data size, 512; spectral width, 2,000 [Hz]; 4 acquisitions; 8.5 min. MRI room temperature was maintained at 21-25 °C. On the next day after the MRI scan, 15O-gas PET was performed in all patients at a SET-3000GCT/M scanner (PET/CT; Shimadzu Corp., Kyoto, Japan). Raw data from 1H-MRS (apodization; 1 Hz and fast Fourier transform) was analyzed by the automatic curve fitting procedure and decomposed into Lorentzian peak components using our custom-made software6. BT in each voxel was calculated from the chemical shift difference between water and N-acetylaspartate signals Δ(H2O – NAA) using the following formula defined by Cady et al: T [°C] = 286.9 – 94*Δ(H2O – NAA)7. After the BT calculation in all voxels, BT map was generated (a typical case in Figure 1a). All PET images were reformatted into the slices coregistered to T2W images with the 5×5-voxel ROIs using Dr.View software (AJS, Tokyo, Japan). In each voxel-pair that was composed of two voxels on the affected and contralateral sides at the corresponding position in rows at the ROIs’ edge (Figure 1b), ΔBT (BT on the affected side – BT on the contralateral side) was calculated. In each PET image, the ratios of the value in the affected hemisphere to that in the contralateral hemisphere was calculated using the same voxel-pair (Figure 1c). Finally, ΔBT and PET ratio were obtained in 5 voxel-pairs. Additionally, the mean values of the 5 voxel-pairs of all data were also calculated in each patient. To define the normal cut-off value, 20 healthy subjects underwent multi-voxel 1H-MRS and 10 healthy subject underwent PET, respectively. In each group, mean of ΔBT, CBF, CBV, CMRO2 or OEF ratio was calculated with defining the left cerebral hemisphere as the affected side.RESULTS
BT maps were successfully generated in all patients. In 175 voxel-pairs (5 voxel-pairs×35 patients), ΔBT significantly correlated with CBV ratio (r=0.57, p<0.0001), CMRO2 ratio (r=0.39, p<0.0001) and OEF ratio (r=0.64, p<0.0001). Also, for the mean values of 5 voxel-pairs in each patient, mean ΔBT correlated with mean CBV (r=0.70, p<0.0001) , mean CMRO2 (r=0.50, p=0.0017) and mean OEF ratio (r=0.78, p<0.0001).DISCCUTION
In chronic ischemia, reduced CBF include the different patho-physiological conditions because the condition can depend on the brain metabolism. By a single-voxel MRS measurement, it has been reported that BT significantly correlated with OEF3 indicating how the cerebral oxygen metabolism maintained. The significant correlation between ΔBT and OEF ratio observed in the present work agreed with the previous result. It might indicate that the BT measurement with a multi-voxel 1H-MRS had the sufficient accuracy comparable to the single-voxel technique.CONCLUSION
BT in the CWM by multi-voxel 1H-MRS correlates with the cerebral hemodynamic abnormalities in patients with unilateral chronic major cerebral artery steno-occlusive disease. The further investigation may validate whether BT can identify the patients at high risk for the stroke recurrence.Acknowledgements
This study was supported in part by Grant-in-Aid for Strategic Medical Science Research (S1491001, 2014-2018) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.References
1.Kluytmans M, van der Grond J, Folkers PJ, Mali WP, Viergever MA. Differentiation of gray matter and white matter perfusion in patients with unilateral internal carotid artery occlusion. J Magn Reson Imaging 1998;8:767-774.
2.Moody DM, Bell MA, Challa VR. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: an anatomic study. AJNR Am J Neuroradiol 1990;11:431-439.
3.Ishigaki D , Ogasawara K , Yoshioka Y, et al. Brain temperature measured using proton MR spectroscopy detects cerebral hemodynamic impairment in patients with unilateral chronic major cerebral artery steno-occlusive disease: comparison with positron emission tomography. Stroke 2009;40:3012-3016.
4.Fujiwara S, Yoshioka Y, Matsuda T, Nishimoto H, Ogawa A, Ogasawara K, Beppu T. Relation between brain temperature and white matter damage in subacute carbon monoxide poisoning. Sci Rep (in press).
5.Karaszewski B, Carpenter TK, Thomas RG, Armitage PA, Lymer GK, Marshall I, et al. Relationships between brain and body temperature, clinical and imaging outcomes after ischemic stroke. J Cereb Blood Flow Metab. 2013;33(7):1083-9.
6.Yoshioka Y, Oikawa H, Ehara S, et al. Noninvasive measurement of temperature and fractional dissociation of imidazole in human lower leg muscles using 1H-nuclear magnetic resonance spectroscopy. J Appl Physiol 2005;98:282-287.
7.Cady EB, D’Souza PC, Penrice J, Lorek A. The estimation of local brain temperature by in vivo 1H magnetic resonance spectroscopy. Magn Reson Med 1995;33:862–867.
Figures