4628
Measure Cerebral Microstructure Changes in Brain Small Vessel Disease Using Diffusion Kurtosis ImagingWenjing Lan1, Shuang Xu1, Yang Liu1, Kaining Shi2, and Lizhi Xie3
1The First Hospital of Jilin University, Changchun, People's Republic of China, 2Philips Healthcare (China), Beijing, People's Republic of China, 3GE Healthcare, MR Research China, Beijing, People's Republic of China
Synopsis
Diffusion tensor imaging (DTI) has been the most commonly used modality among diffusion MRI methods in the studies of ageing and development in the current study, we investigated diffusional modifications arising from brain small vessel disease, as compared with age and educational level matched healthy controls. Diffusion kurtosis imaging (DKI) was applied throughout the study, which is a recent novel extension of DTI to provide additional metrics quantifying non-Gaussianity of water diffusion in brain tissues.
Purpose
To observe modifications in cerebral microstructure in brain small vessel disease using magnetic resonance imaging (MRI) diffusion kurtosis imaging (DKI) and to provide pathogenesis information of this disease from the perspective of radiography.Material and Methods
Results and Discussions
There was no statistically significant difference in FA value of bilateral thalamas, the posterior limb of the internal capsule, corona, centrum ovale or left basal ganglia between two groups (P>0.05) . FA value of right basal ganglia in the patient group was significantly decreased than that of the control group (P<0.05) (Figure 1) . There was no significant difference in MK value of bilateral basal ganglia, thalamas, the posterior limb of the internal capsule, the location beside lateral ventrical posterious cornu, or the callosum between two groups (P>0.05) . MK value of left corona radiata ,bilateral centrum ovale and pons of the patient group was significantly decreased than that of the control group (P<0.05) (Figure 2). Moreover, no significant difference was observed in MD value of bilateral basal ganglia, the posterior limb of the internal capsule, the location beside lateral ventrical anterior and posterious cornu, callosum, right thalamas , centrum ovale and pons between two groups (P>0.05) . MD value of bilateral corona radiata ,left thalamas and centrum ovale of the patient group was significantly decreased than that of the control group (P<0.05) (Figure 3) . The conventional diffusion parameters were estimated using the mono-exponential model, where the values derived depended on the selection of b-values. As an extension of DTI model, DKI required at least two non-zero b values in more than 15 independent directions. Using a second-order polynomial model, DKI would provide a b-value-independent estimation of the diffusion and kurtosis parameters. Therefore, DKI could be an ideal technique for estimating the restricted diffusion process in vivo, especially in detecting the pathological alterations in neural tissues. Conclusion
Acknowledgements
No acknowledgement found.References
[1] Lanzafame S et al, 2016, MedPhys, 43(5):2464
[2] Coutu JP et al, 2014, Neurobiol Aging, 35(6)1421-21
[3] Jiajia Zhu et al, 2015, Neuroimage Clin,7: 170–176
Figures
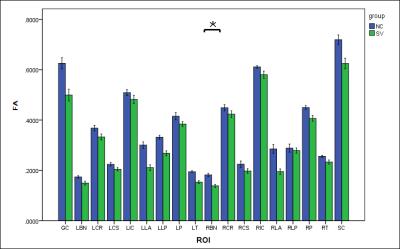
Figure.1 Comparison of the FA values
of the left and right cerebral white matters in basal ganglia, thalamus, corona
radiata, centrum ovale, and the location beside lateral ventrical , pons and
callosum.
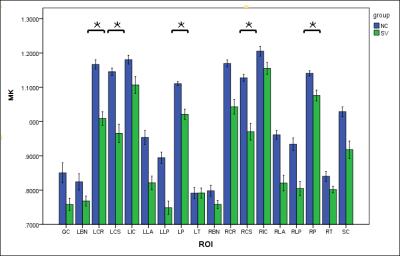
Figure.2 Comparison of MK values of left
and right cerebral white matter in basal ganglia, thalamus,
corona radiata, centrum ovale, and the location beside lateral ventrical , pons
and callosum.

Figure.3 Comparison
of the MD values of left and right cerebral white matter in basal ganglia, thalamus,
corona radiata, centrum ovale, and the location beside lateral ventrical , pons
and callosum.