4626
FEAST based Arterial Transit Time Measurement using Pseudo Continuous Arterial Spin Labeling in Patients with Sickle Cell Disease1Department of Electrical Engineering, University of Southern California, Los Angeles, CA, United States, 2Department of Biomedical Engineering, University of Southern California, Los Angeles, CA, United States, 3Radiology, Children's Hospital of Los Angeles, Los Angels, CA, United States, 4Section of Hematology, Children's Hospital of Los Angeles, Los Angels, CA, United States, 5Division of Cardiology, Children's Hospital of Los Angeles, Los Angels, CA, United States
Synopsis
Sickle cell disease (SCD) is a genetic blood disorder with a high prevalence of cerebral vasculopathy and stroke. Arterial Transit Time (ATT) refers to the time it takes blood to flow from the labeling plane, to the vascular imaging compartment and reflects cerebrovascular impairment in SCD. We used pseudo continuous arterial spin labeling to measure ATT with flow encoding arterial spin tagging technique in patients with SCD and ethnicity matched, healthy controls. Our findings demonstrated sensitivity of ATT to vasculopathy.
Introduction
Sickle cell disease (SCD) is a genetic blood disorder with a high prevalence of cerebral vasculopathy and stroke. Arterial Transit Time (ATT) refers to the time it takes blood to flow from the labeling plane, to the vascular imaging compartment and may be a potential physiologic parameter that reflects cerebrovascular impairment in SCD. In this study, we used pseudo continuous arterial spin labeling (PCASL) to measure ATT with flow encoding arterial spin tagging (FEAST)1 in patients with SCD and ethnicity matched, healthy controls. Our hypothesis was that patients with SCD would exhibit shorter ATT due to elevated cerebral blood flow (CBF) and velocity2.Methods
In FEAST, ATT was derived based on a two-compartment perfusion model3. The perfusion signal, ΔM, acquired without bipolar gradients, contains two sources of signal contribution: vascular and micro-vascular blood flow. By applying a velocity encoding (venc, 5cm/s during a T2 preparation module) gradient, the vascular signal is nulled and only the micro-vascular signal, ΔM′, remains and can be represented by the following equations$$ \Delta M=\dfrac{2T_{1\alpha}M_0f\alpha}{\lambda}\left[e^{-\omega/T_{1\alpha}} -e^{-(\tau+\omega)/T_{1\alpha}} \right]$$ (1) $$ \Delta M=\dfrac{2T_{1\alpha}M_0f\alpha}{\lambda}\left[e^{-\delta/T_{1\alpha}} -e^{-(\tau+\omega)/T_{1\alpha}} \right]$$ (2),
where f is CBF, T1a is the longitudinal relaxation time of blood (1850 ms), M0 is the equilibrium magnetization of brain, α is labeling efficiency, λ is blood/tissue water partition coefficient (0.9), w is post labeling delay (1525 ms), τ is labeling duration (1150 ms) and δ is arterial transit time. After measuring ΔM and ΔM′, we then solved for δ. We performed motion correction using MCFLIRT4 and used blood flow velocity acquired by phase-contrast (PC) MRI to estimate α for each subject5. All studies were conducted under an approved IRB protocol using a 3T Philips MRI scanner with an 8-channel head coil.
Results
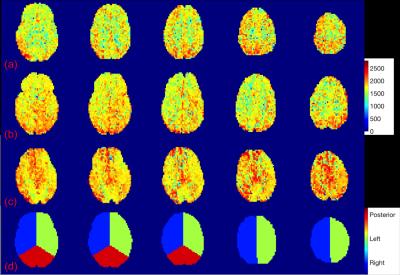
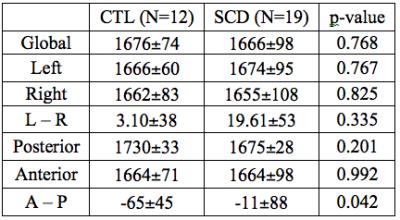
We acquired PCASL MR images for 31 subjects, including 19 SCD patients (7M, 12F, age: 21.4±9.7) and 12 healthy controls (3M; 9F; age: 33.6±14.4). All patients had normal MR angiograms except one patient with bilateral anterior cerebral artery stenosis. Cerebral artery territories were manually masked into left/right hemisphere, anterior and posterior areas for regional ATT analysis shown in Fig. 1 (d). In Fig. (a), (b) and (c), the regional ATT maps are shown for a healthy control subject, a relatively healthy patient with SCD, and the SCD patient having severe bilateral proximal anterior cerebral artery stenosis. Overall, there was no difference in regional ATT between the groups (1676±74 ms and 1666±98 ms for controls and SCD, respectively). No asymmetry was detected between left or right hemisphere in any patient. The control group showed a significant posterior-anterior ATT difference compared with patients with SCD. In addition, resting CBF estimated by PC was positively associated with ATT (p<0.04) in controls but had no significant association in patients with SCD. However, ATT was markedly prolonged (1838 ms) in the patient with severe vasculopathy (Fig. 1 (c)), particularly in the anterior territory circulation (1843 ms) compared with posterior territory (1808 ms), demonstrating sensitivity of ATT to vasculopathy.Discussion
In this work, we demonstrate that it is possible to quantify ATT in patients SCD using FEAST ASL. Our ATT estimates are in excellent agreement with the literature, and match expected physiology in healthy controls6 and pathophysiology in individual patients (Fig. 1). We expected ATT to decrease in patients with SCD due to elevated CBF. Additionally, we postulated that there might be right-left asymmetries in ATT due to right-left CBF asymmetries in these patients with SCD7. We were unable to observe either of these effects in our patient population. By the central volume theorem, increased CBF in the presence of normal ATT implies that SCD patients must have increased cerebral blood volume. ATT was sufficiently sensitive to detect major vessel stenosis, but further studies are ongoing to determine whether ATT and CBV may be a useful prognostic biomarker of micro-vascular damage in SCD.Acknowledgements
This work was supported by the National Heart Lung and Blood Institute U01HL117718.References
1. Wang J. et al., “Arterial transit time imaging with flow encoding arterial spin tagging (FEAST),” Magnetic Resonance in Medicine, vol. 50, no. 3, pp. 599-607, 2003.
2. Bush A. et al., “Determints of resting cerebral blood flow in sickle cell disease,” American J of Hematology, 2016.
3. Alsop D. et al., “Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow,” J Cereb Blood Flow Metab, vol. 16, pp. 1236-1249 1996.
4. Mark J. et al., “Improved optimization for the robust and accurate linear registration and motion correction of brain images,” Neuroimage, vol. 12, no. 2, pp. 825-841, 2002.
5. Chai Y. et al., “An experimental investigation of labeling efficiency for pseudo-continuous arterial spin labeling labeling,” Biomedical Imaging (ISBI), 2016 IEEE 13th International Symposium on, pp. 1101-1104, IEEE, 2016.
6. Chen Y. et al., “Comparison of arterial transit times estimated using arterial spin labeling,” Magnetic Resonance Materials in Physics, Biology and Medicine, vol. 25, no. 2, pp. 135-144, 2012.
7. van den T. et al., “Cerebral blood flow measurement in children with sickle cell disease using continuous arterial spin labeling at 3.0-Tesla MRI,” Stroke, vol. 40, no. 3, pp. 795-800, 2009.
Figures