4620
Clinical application of Half Fourier Acquisition Single Shot Turbo Spin Echo (HASTE) imaging with multiband (MB) excitation and PINS refocusing pulses1Donders Institute for Brain, Cognition and Behaviour, Radboud University Nijmegen, Nijmegen, Netherlands, 2Department of Neurology, Radboud University Medical Centre, Nijmegen, Netherlands, 3Department of Radiology and Nuclear Medicine, Radboud University Medical Centre Nijmegen, Nijmegen, Netherlands, 4Erwin L. Hahn Institute for Magnetic Resonance Imaging, University Duisburg-Essen, Essen, Germany
Synopsis
In this abstract, we demonstrate a clinical application of a MB-PINS-HASTE sequence with TRAPS and compare it to a standard HASTE protocol which is used in daily clinical practice. The modified MB-PINS-HASTE sequence offers the possibility to overcome limitations related to its typically high energy deposition and can accelerate the acquisition by acquiring several slices simultaneously. The reconstructed images show good diagnostic quality for the evaluation of the overall brain and CSF spaces in patients with small vessel disease. MB-PINS HASTE therefore offers the possibility of using ultrafast spin-echo imaging to acquire anatomical T2-weighted images and follow dynamic signal changes.
Purpose
Offering a pure T2-weighted contrast, high sensitivity and low artifact levels, rapid acquisitions with ultrafast spin-echo sequences have found wide-spread application. Small vessel disease shows a T2-contrast1 and HASTE can probe the overall aspect of the brain and CSF spaces. As a single-shot spin echo imaging sequence with a long train of refocusing pulses, HASTE suffers from high power deposition which particularly limits its use at high resolutions and high field strengths, particularly if combined with acceleration techniques such as standard multiband (MB)/ simultaneous multislice2 (SMS) imaging. In this abstract, this limitation is overcome by combining MB excitation pulses with low SAR PINS-refocusing pulses3 modulated with TRAPS4. Because of its speed, HASTE is clinically mainly applied for scanning uncooperative or young patients without the need for sedation. To evaluate the diagnostic value, the MB-PINS-HASTE sequence is compared to a standard HASTE protocol, which is used in daily clinical practice.Methods
Standard MB excitation pulses and PINS refocusing pulses were incorporated into a standard Siemens HASTE sequence. In order to make use of the Siemens slice GRAPPA reconstruction WIP, a FLASH reference scan was implemented at the beginning of the sequence. Blipped CAIPIRINHA5 is used to shift individual slices in the z-direction improving image reconstruction while TRAPS is implemented to decrease the SAR. Whole brain data were acquired on a Siemens 3T Prisma system from 6 patients. The standard clinical HASTE protocol parameters were: TR/TE=700/92ms, TAcq=18s, matrix 320x243, GRAPPA2, PF 5/8, 26slices, 20%gap, 0.8x0.8x5.0mm res. The MB-PINS-HASTE acquisition parameters were: TR/TE=1700(2500)/60ms, TAcq=15(22)s, matrix 192x192, FatSat, PF 4/8, TRAPS 60deg, MB4, CAIPI3, 36slices, 0%gap, 1.1x1.1x3.0 mm res. All protocols were acquired in transversal, sagittal and coronal orientations.Results
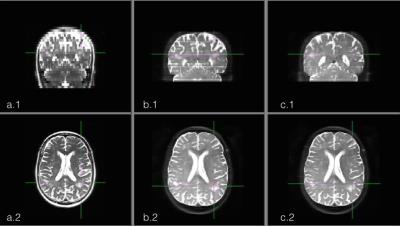
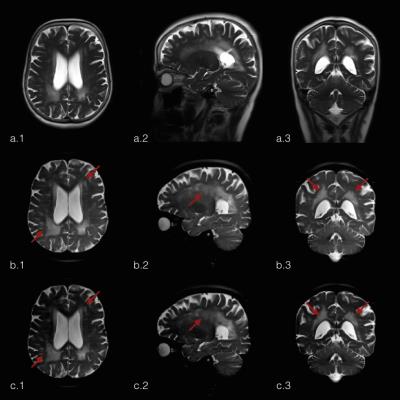
Figure 1 shows the difference in effective resolution between the standard HASTE and the MB-PINS-HASTE protocols. The coverage of the MB-PINS-HASTE protocols is less owing to the thinner slices, but the images have a smaller slice thickness and no slice gap. In general, the MB-PINS HASTE protocols show sufficient diagnostic image quality for the evaluation of the overall brain and CSF spaces. There are no significant artifacts caused by the acquisition or reconstruction influencing the image quality. Furthermore, white matter changes are clearly visible and better demarcated than on the standard HASTE sequence for the patient shown in figure 2 (indicated by the arrows).Discussion
It is to be noted that the two protocols differ in effective TE, in-plane resolution and slice thickness, although the pixel volume is virtually identical. This is due to limitations of the current implementation of the in-plane acceleration on the modified sequence. Higher MB factors should in principle be attainable resulting in higher accelerations. With MB-PINS HASTE, cerebral white matter changes could be more accurately detected and evaluated than with the standard HASTE while scanning time could even be decreased for one of the MB-PINS-HASTE protocols. This could save (young) patients additional MRI scans under sedation. For further evaluation of the diagnostic accuracy, the MB-PINS HASTE sequence needs to be compared to a routinely used FLAIR or T2-TSE acquisition in a larger study population.Conclusion
MB-PINS HASTE enables the acquisition of slice accelerated highly T2-weighted images and provides good diagnostic image quality. It offers the possibility of using ultrafast spin-echo imaging to acquire anatomical T2-weighted images and follow dynamic signal changes. With the implementation of TRAPS, future implementation at 7T should be possible.Acknowledgements
No acknowledgement found.References
1Haller S et al, 2013, Acra Neuropathol Commun 1.1:1
2Larkman DJ et al, 2001, J Magn Reson Img 13:313-317
3Norris DG et al, 2011, Magn Reson Med 66:1234-1240
4Hennig et al, 2003, Magn Reson Med 49:527-535
5Setsompop K et al, 2012, Magn Reson Med 67:1210-1224
Figures