4608
Resting Functional Connectivity and Anatomical Basis in Patients with Hepatic Myelopathy After Transjugular Intrahepatic Portosystemic Shunt1Department of Radiology, Xijing Hospital, Fourth Military Medical University, Xi'an, People's Republic of China, 2National University of Defense Technology College of Mechatronic Engineering and Automation, Changsha, People's Republic of China, 3Xijing Hospital of Digestive Diseases, Xijing Hospital, Fourth Military Medical University, Xi'an, People's Republic of China
Synopsis
We investigated whether motor system in the brain shows altered functional connectivity and what the anatomical basis behind it is in patients with hepatic myelopathy (HM) after transjugular intrahepatic portosystemic shunt (TIPS), a rare and likely overlooked complication in chronic liver disease patients with portosystemic shunts characterized by severe and mostly irreversible neurologic symptoms. HM patients exhibited hypoconnectivity between the right SMA and right insula, which revealed decreased gray matter volume. The positive correlation was observed between the strength of this connectivity and folic acid level in HM patients, which could discriminate HM from Non-HM with a high level of accuracy.
INTRODUCTION: As a rare and likely overlooked complication in chronic liver disease patients with portosystemic shunts, hepatic myelopathy (HM) is characterized by severe and mostly irreversible neurologic symptoms and may be secondary to surgical procedures such as transjugular intrahepatic portosystemic shunt (TIPS).1 HM patients, pronouncedly presenting with a progressive symmetric spastic paraparesis of the lower limbs,2 are usually resistant to medial therapy and ultimately wheelchair-bound with dramatically decreased quality of life. However, routine examinations could not be useful for diagnosing HM, because their results are usually normal or non-specific abnormal in patients with HM. The diagnosis of HM, established on clinical grounds after excluding other possible causes of spastic paraparesis,3 is a difficult clinical problem due to largely unknown neuropathological underpinnings behind HM and a lack of specific biomarkers for this devastating disorder. Cerebral functional magnetic resonance imaging (fMRI) that detects slight alteration of the regional and network-level brain activity might be helpful in the early diagnosis of HM, which promisingly helps to guide the treatments and improve the outcome of patients with HM. We aimed to investigate: (1) whether motor system in the brain of HM shows altered patterns of functional connectivity, (2) what the anatomical basis behind it is, and (3) the potential clinical significance of these alterations.
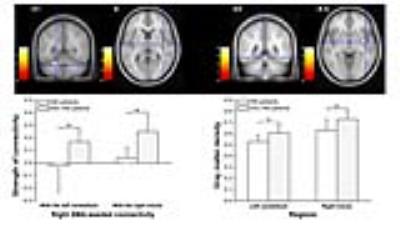
METHODS: After undergoing careful medical history taking, physical examination, and imaging and laboratory examinations, 23 patients with HM and 23 matched patients without HM after TIPS were enrolled in the current study. Twenty-four demographically matched healthy controls (HCs) were recruited. High-resolution T1-weighted structural data were acquired using the 3D magnetization-prepared rapid gradient echo (3D MPRAGE) sequence and echo planar imaging (EPI) sequence. We included cortical and subcortical motor areas involving motor and motor control as seed regions for functinal connectivity analysis.4 Coordinates of these regions were from activation likelihood estimation meta-analyses (primary motor cortex: Hardwick et al.5, dorsolateral premotor cortex: Witt et al.6; supplementary motor area (SMA): Rehme et al.7; pre-SMA: Keuken et al.8; putamen: Hardwick et al.5). Then, we performed gray matter density analysis using the Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL) procedure.9 RESULTS: When comparing HM and Non-HM, we found weaker right SMA-seeded functional connectivity with bilateral insula and thalamus, left cerebellum and middle temporal gyrus, and right middle cingulate gyrus and midbrain in HM patients (Figure 1). Among the regions showing altered connectivity with the right SMA, the right insula and left cerebellum just revealed decreased volume in HM patients relative to Non-HM patients (Figure 2). Furthermore, the strength of right SMA-seeded functional connectivity was positively correlated with folic acid level in HM patients (r = 0.59, P = 0.04) (Figure 3). In addition to clinical correlation, the strength of right SMA-seeded functional connectivity also showed the best diagnostic value of distinguishing HM patients from Non-HM patients (area under curve: 0.90; 95%CI: 0.81, 1.00) (Figure 3).
DISCUSSION: In the clinical setting, HM is characterized by progressive and irreversible spastic paraparesis without sensorial deficit and sphincteric impairment. Increased tone and hyperreflexia are the most common findings, and upper extremities are minimally affected. Since motor system, especially upper motor neurons, is mostly involved in HM patients, we explored whether motor system in the brain of HM shows altered functional connectivity and the clinical implications of functional imaging features. In the current study, notably, HM patients exhibited hypoconnectivity between the right SMA and right insula, which revealed decreased gray matter volume. Moreover, the positive correlation was observed between the strength of this connectivity and folic acid level in HM patients, which could discriminate HM from Non-HM with a relative high level of accuracy. Kurth et al. found the mid-posterior insula as a sensorimotor region based on the activation-likelihood-estimation (ALE) meta-analysis,10 which is supported by the evidence of a network-based approach using the insula parcellated on the basis of task-evoked coactivation, resting-state functional connectivity, and gray matter structure.11 The interaction within the motor system, the crosstalk between the brain and spinal cord, and the linkage between this neural regulation and the onset of HM will deserve further investigation in the future, particularly focusing on the brain and the cerebrospinal interaction.
CONCLUSION: In conclusion, this study demonstrates the HM-specific functional connectivity between the right SMA and insula with an anatomical basis. Furthermore, our study also reveals this aberrant connectivity’s correlation with laboratory findings, as well as its diagnostic value to discriminate HM from Non-HM patients. Our findings might help to predict and prevent HM at an early stage after TIPS, as well as improve the outcome of HM.
Acknowledgements
No acknowledgement found.References
1. Zhao H, Liu F, Yue Z, et al. Evaluation of mid- and long-term efficacy of shunt limiting for hepatic myelopathy after transjugular intrahepatic portosystemic shunt. Clin Res Hepatol Gastroenterol. 2016;40(4):440-446.
2. O'Brien J, Staples C, Florin T. Trouble with a shunt: alcohol and spastic paraparesis. Hepatic myelopathy. Gastroenterology. 2010;139(4):1099, 1428.
3. Mendoza G, Marti-Fàbregas J, Kulisevsky J, et al. Hepatic myelopathy: a rare complication of portacaval shunt. Eur Neurol. 1994;34(4):209-212.
4. Pool EM, Rehme AK, Eickhoff SB, et al. Functional resting-state connectivity of the human motor network: differences between right- and left-handers. Neuroimage. 2015;109:298-306.
5. Hardwick RM, Rottschy C, Miall RC, et al. A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage. 2013;67:283-297.
6. Witt ST, Laird AR, Meyerand ME. Functional neuroimaging correlates of finger-tapping task variations: an ALE meta-analysis. Neuroimage. 2008;42:343-356.
7. Rehme AK, Eickhoff SB, Rottschy C, et al. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. Neuroimage. 2012;59:2771-2782.
8. Keuken MC, Muller-Axt C, Langner R, et al. Brain networks of perceptual decision-making: an fMRI ALE meta-analysis. Front Hum Neurosci. 2014;8:445.
9. Zeng LL, Liu L, Liu Y, et al. Antidepressant treatment normalizes white matter volume in patients with major depression. PLoS One. 2012;7(8):e44248.
10. Kurth F, Zilles K, Fox PT, et al. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214(5-6):519-534.
11. Kelly C, Toro R, Di Martino A, et al. A convergent functional architecture of the insula emerges across imaging modalities. Neuroimage. 2012;61(4):1129-1142.
Figures