4602
Low-frequency Visual Entrainment Enhances Bilateral Resting-state fMRI Connectivity in Primary Sensory Cortices1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, Hong Kong, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, Hong Kong, 3Department of Physics and Materials Science, City University of Hong Kong, Hong Kong, Hong Kong
Synopsis
Entrainment is known to alter or synchronize brain rhythm and may enhance task performance. However, whether and how sensory entrainment may modulate the long-range brain functional networks are unknown. We investigated the effects of frequency-dependent visual entrainment on resting state functional connectivity in distinct sensory cortical networks. Our findings provide the first and direct evidence that only low frequency visual entrainment can modulate the long-range non-visual sensory networks. They suggest that the entrained neural oscillation at low frequency can actively contribute to the long-range interactions between primary sensory cortical functional networks that underlie the brainwide connectivity measured by resting-state fMRI.
Purpose
Sensory entrainment refers to the use of external periodic stimuli to synchronize neural activity in the sensory cortices1,2. Previous studies have demonstrated that neural oscillations in sensory cortices can be temporally entrained by external stimulation3-5 and may influence sensory perceptions6,7, performances8 and mental state8. The frequency characteristic of the entrainment has been suggested to play a role in sensory perception and performance. For example, delta-band (1-4Hz) entrainment is related to perceive non-speech-specific acoustic rhythm, while alpha-band (8-12Hz) entrainment may enhance visual perception. Resting-state fMRI (rsfMRI) maps brain functional networks9 and may reflect sensory perceptions and performances. However, whether and how sensory entrainment may modulate the long-range brain functional networks are unknown. In this study, we investigated the effects of frequency-dependent visual entrainment on rsfMRI functional connectivity in distinct sensory cortical networks.Methods
Visual Stimulation
Adult male Sprague-Dawley rats (n=16, 9 weeks old, 350-400g) were maintained at 1% isoflurane during rsfMRI experiments. Binocular visual stimulation was delivered through an optical fiber placed 3 cm in front and along the midline of the eyes. 10 minutes continuous 1Hz (n=8) or 10Hz (n=8) stimulation (10% duty cycle, 1020 lux which is comparable to normal office lighting) was used as visual entrainment after the baseline rsfMRI acquisitions.
rsfMRI Protocol and Data Analysis
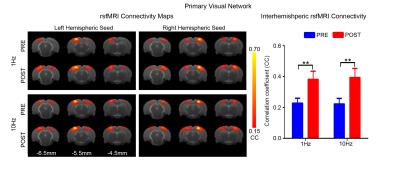
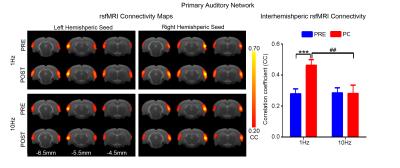
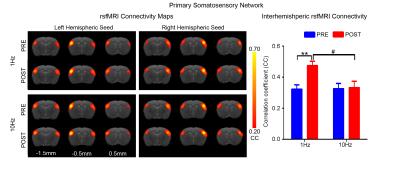
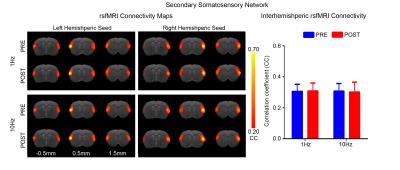
rsfMRI acquisitions were performed at 7T with paradigm in Fig. 1 using GE-EPI (FOV=32×32mm2, matrix=64×64, α=56°, TE/TR=20/750ms, 16 contiguous slices with 1mm thickness) before and after visual stimulation. After standard preprocessing procedures, seed-based analysis10 was applied to quantify rsfMRI connectivity between bilateral primary visual cortex (V1), auditory cortex (A1), somatosensory cortex (S1) and secondary somatosensory cortex (S2). The significance of difference in functional connectivity at defined networks was calculated by two-way ANOVA statistical analysis.
Results
Figs. 2 - 5 show the rsfMRI connectivity maps of bilateral V1, A1, S1, and S2, respectively, before and after 1Hz and 10Hz visual entrainment. The correlation coefficient values (CC-values) of V1, A1, S1 and S2 networks before and after stimulation were extracted and compared. The bilateral V1 functional connectivity increased significantly after both low and high frequency visual entrainment (Figs. 2 – 3), indicating that acute sensory stimulus can induce functional connectivity increase in the corresponding sensory region. Low frequency entrainment strengthened the functional connectivity in other primary sensory networks, including S1 and A1, but not high frequency entrainment. These results imply the involvement of cross-modal interaction among V1, A1 and S1, and suggest such interaction is promoted under low frequency stimulus. Meanwhile, no observable functional connectivity changes were detected in S2.Discussion and Conclusion
This study revealed the specific influences of
visual entrainment on functional connectivity in sensory cortical networks. The bilateral V1 functional connectivity was
found to be enhanced by both 1Hz and 10Hz visual entrainment. In contrast, low
frequency, not high frequency, visual entrainment enhanced the bilateral A1 and
S1 functional connectivity. Although the cross-modal influence has been well
demonstrated in numerous studies where visual perception was shown to modulate auditory
and somatosensory cortical processing11,12, our findings provide the
first and direct evidence that only low frequency visual entrainment can
modulate the long-range non-visual sensory networks. These results suggest that
the entrained neural oscillation at low frequency can actively contribute to the
long-range interactions between primary sensory cortical functional networks
that underlie the brainwide connectivity measured by resting-state fMRI.
Rhythmic oscillations are critical for communication between sensory networks7,8. Previous electrophysiological recording from rat visual cortex showed periodic signals influence neural activity over a distance in cortex in a greater extent than aperiodic signals, especially in low frequency oscillations13,14. Conduction delay between areas was suggested to be an important factor to contribute cortical network interactions15,16. Low frequency oscillations have a rather long time window for information processing which allows synchronizing distant brain regions with large conduction delay between areas15,17,18.
Recent, brain oscillation was demonstrated to be phase-locked by applying rhythmic visual stimulation at alpha frequency19. Alpha oscillation was thought as a prominent rhythm of visual system, 10Hz visual stimulation had been shown to enhance occipital-parietal alpha power20,21. Furthermore, resonance of steady-state potential was found to be induced at 10Hz photic stimuli20, which matched with enhanced V1 functional connectivity at 10Hz entrainment in our study.
In summary, low and high frequency visual entrainment induced V1 functional connectivity increased. In addition, the enhanced functional connectivity was not only confined within visual cortex, but also observed in primary sensory cortical networks cross-modally after low frequency visual entrainment. This suggested that low frequency oscillations, temporally realigned by low frequency entrainment, contribute to long-range cortical networks modulation.
Acknowledgements
No acknowledgement found.References
1. Gross, J., Hoogenboom, N., Thut, G., Schyns, P., Panzeri, S., Belin, P., & Garrod, S. (2013). Speech rhythms and multiplexed oscillatory sensory coding in the human brain. PLoS Biol, 11(12), e1001752.
2. Lakatos, P., Musacchia, G., O’Connel, M. N., Falchier, A. Y., Javitt, D. C., & Schroeder, C. E. (2013). The spectrotemporal filter mechanism of auditory selective attention. Neuron, 77(4), 750-761.
3. Taylor-Clarke, M., Kennett, S., & Haggard, P. (2002). Vision modulates somatosensory cortical processing. Current Biology, 12(3), 233-236.
4. Besle, J., Schevon, C. A., Mehta, A. D., Lakatos, P., Goodman, R. R., McKhann, G. M., ... & Schroeder, C. E. (2011). Tuning of the human neocortex to the temporal dynamics of attended events. The Journal of Neuroscience, 31(9), 3176-3185.
5. Montemurro, M. A., Rasch, M. J., Murayama, Y., Logothetis, N. K., & Panzeri, S. (2008). Phase-of-firing coding of natural visual stimuli in primary visual cortex. Current biology, 18(5), 375-380.
6. Buzsáki, György, and Andreas Draguhn. "Neuronal oscillations in cortical networks." science 304.5679 (2004): 1926-1929.
7. Buzsaki, Gyorgy. Rhythms of the Brain. Oxford University Press, 2006.
8. Tang, H. Y. J., Riegel, B., McCurry, S. M., & Vitiello, M. V. (2016). Open-Loop Audio-Visual Stimulation (AVS): A Useful Tool for Management of Insomnia?. Applied psychophysiology and biofeedback, 41(1), 39-46.
9. Fox, M. D., & Raichle, M. E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience, 8(9), 700-711.
10. Zhou, I. Y., Liang, Y. X., Chan, R. W., Gao, P. P., Cheng, J. S., Hu, Y., ... & Wu, E. X. (2014). Brain resting-state functional MRI connectivity: morphological foundation and plasticity. NeuroImage, 84, 1-10. 11. Eckert, M. A., Kamdar, N. V., Chang, C. E., Beckmann, C. F., Greicius, M. D., & Menon, V. (2008). A cross-modal system linking primary auditory and visual cortices: Evidence from intrinsic fMRI connectivity analysis. Human brain mapping, 29(7), 848-857.
12. Kayser, C., Petkov, C. I., & Logothetis, N. K. (2008). Visual modulation of neurons in auditory cortex. Cerebral Cortex, 18(7), 1560-1574.
13. Shang, C. F., Dan, Y., Poo, M. M., & Wang, Z. (2011). Periodic stimulation induces long-range modulation of cortical responses and visual perception. The Journal of physiology, 589(13), 3125-3133.
14. Notbohm, A., Kurths, J., & Herrmann, C. S. (2016). Modification of brain oscillations via rhythmic light stimulation provides evidence for entrainment but not for superposition of event-related responses. Frontiers in human neuroscience, 10.F
15. Von Stein, A., & Sarnthein, J. (2000). Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. International Journal of Psychophysiology, 38(3), 301-313.
16. Kopell, N., Ermentrout, G. B., Whittington, M. A., & Traub, R. D. (2000). Gamma rhythms and beta rhythms have different synchronization properties. Proceedings of the National Academy of Sciences, 97(4), 1867-1872.
17. Canolty, R. T., & Knight, R. T. (2010). The functional role of cross-frequency coupling. Trends in cognitive sciences, 14(11), 506-515.
18. Siegel, M., Donner, T. H., & Engel, A. K. (2012). Spectral fingerprints of large-scale neuronal interactions. Nature Reviews Neuroscience, 13(2), 121-134.
19. De Graaf, T. A., Gross, J., Paterson, G., Rusch, T., Sack, A. T., & Thut, G. (2013). Alpha-band rhythms in visual task performance: phase-locking by rhythmic sensory stimulation. PloS one, 8(3), e60035.
20. Herrmann, C. S. (2001). Human EEG responses to 1–100 Hz flicker: resonance phenomena in visual cortex and their potential correlation to cognitive phenomena. Experimental brain research, 137(3-4), 346-353.
21. Foxe, J. J., & Snyder, A. C. (2011). The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Frontiers in psychology, 2, 154.
Figures