4601
Associations between clinical risk factors, neurocognitive performance and brain activity in survivors of childhood Acute Lymphoblastic Leukemia treated on a chemotherapy-only protocol1Diagnostic Imaging, St Jude Children's Research Hospital, Memphis, TN, United States, 2Psychology, St Jude Children's Research Hospital, Memphis, TN, United States, 3Oncology, St Jude Children's Research Hospital, Memphis, TN, United States
Synopsis
ALL survivors treated with chemotherapy alone remain at elevated risk for neurocognitive impairment. We used fMRI to investigate associations between clinical risk factors, neurocognitive performance and brain activity in survivors of ALL. Ninety-two survivors completed Verbal and Object N-back tasks during fMRI at end of therapy. Measures of working memory were completed as part of a neurocognitive evaluation. Working memory-related brain activation was associated with important clinical risk factors and neurocognitive performance. The pattern of behavioral and imaging responses provides evidence for both compromised and compensatory changes in regional brain function. fMRI may help in selecting patients for remedial intervention.
Purpose
Acute lymphoblastic leukemia (ALL) represents the largest diagnostic group of survivors of childhood cancer1,2. Even when treated with chemotherapy alone, these survivors remain at high risk for neurocognitive impairment, particularly in attention and working memory3,4. Younger age at diagnosis, female gender, and treatment intensity are known risk factors for cognitive deficits in ALL survivors5,6. In this study, we used functional magnetic resonance imaging (fMRI) in a large cohort of survivors of ALL treated with a contemporary chemotherapy-only protocol to investigate associations among clinical risk factors, neurocognitive performance and brain activity.Methods
This study was approved by the IRB and written informed consent was obtained from all participants and/or their parents. Eligible survivors included patients enrolled in the St. Jude Total XVI study, a chemotherapy-only protocol (no cranial radiation) lasting 2.5 years, and with no preexisting neurologic morbidity. Functional imaging was obtained for 92 survivors of ALL (48.4% females; age at exam=13.6± 3.7 years) at the end of therapy. Working memory performance was evaluated based on measures of attention and working memory completed as part of a neurocognitive evaluation in the clinic. Imaging was performed on 3T scanners (Trio or Skyra, Siemens, Malvern, PA) using a single-shot T2*-weighted EPI pulse sequence (TR = 2.06 s, TE = 30 ms, FOV = 192 mm, matrix = 64 x 64, slice thickness = 3.5 mm). Survivors completed Verbal and Object N-back tasks administered at three working memory loads (0, 1 and 2-back). Image data analysis was performed using Statistical Parametric Mapping software (SPM12, Wellcome Institute of Neurology, London, UK). Functional images from each subject were realigned to correct for inter-scan head motion, normalized to the Montreal Neurological Institute brain template and smoothed with a Gaussian kernel of 6-mm full width at half-maximum. The smoothed and normalized images were resampled to 2-mm isotropic resolution. Statistical contrasts were calculated to identify working memory-related networks. Contrast images from each survivor were then used as variables in second-level, random-effect analyses to identify patterns of brain activation. Clinical variables and neurocognitive scores were entered as covariates to identify areas correlated with brain activity. Group differences were tested with a two-sample t-test. Cluster-level analysis was conducted with threshold p<0.001 (uncorrected for multiple comparisons) and minimum cluster size of 5 voxels. The threshold for significance was p<0.05, FWE corrected for multiple comparisons at the voxel-level. Descriptive statistics were calculated using R software (http://cran.r-project.org/) for demographic characteristics and task performance during clinic neurocognitive testing (raw scores and standardized scores) and fMRI. Correlations were tested using Pearson’s test.Results
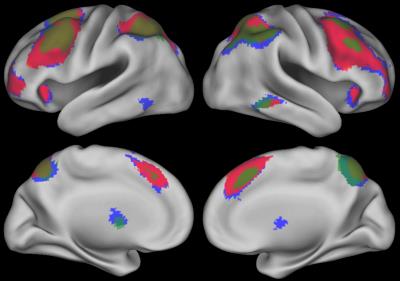
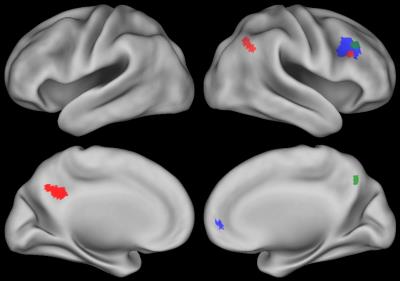
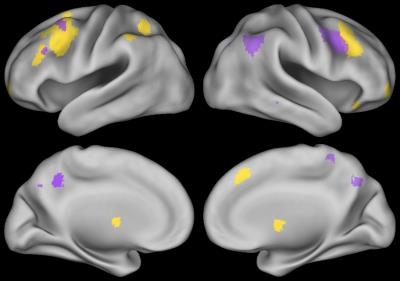
N-back performance during fMRI is summarized in Table 1. The N-back task activated an extensive fronto-parietal network including bilateral insula and anterior cingulate cortex (ACC) (Figure 1, 2-back > 0-back). Brain activation in some regions was correlated with neurocognitive performance during the N-back task are shown in Figure 2 (2-back > 0-back). Decreased brain activity in the dorsolateral prefrontal cortex (DLPFC) and the ACC was significantly associated with lower performance on behavioral measures of decision speed and increased omission errors. Increased activity in areas including the right parietal and left posterior cingulum was significantly correlated with lower working memory performance measured in the clinic. Brain activation in some areas during the N-back task was also associated with demographic factors (Figure 3, 2-back > 0-back). Females had greater activation than males, and activation was lower in children who were younger at ALL diagnosis.Discussion
ALL survivors with poor working memory test performance showed decreased brain activation in areas including the right ACC and the right DLPFC suggesting compromised function in those areas. In contrast, activity increased in right parietal cortex and left posterior cingulum in patients with lower working memory performance, suggestion compensatory function in those areas; however this mechanism seems to be failing. Decreased brain activation was correlated with younger age in areas of the working memory brain network. The increased brain activity in females compared to males could be due to the effect of sex hormones7 or other factors like treatment.Conclusion
Working memory-related brain activation was associated with important clinical risk factors and with neurocognitive performance. The overall pattern of behavioral and imaging results might be explained by altered or compensatory engagement of neural systems to try to maintain task performance despite neurocognitive impairment. Analysis of fMRI responses by medical and demographic factors may help to clarify the pathophysiology of neurocognitive deficits often seen in ALL survivors. Thus, fMRI may help in selecting patients for remedial intervention based on neural phenotypes.Acknowledgements
We thank Patrick Hanby for his help with this project.References
1. Hunger SP, Lu X, Devidas M, et al: Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children's oncology group. J Clin Oncol 30:1663-9, 2012
2. Pui CH, Campana D, Pei D, et al: Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med 360:2730-41, 2009
3. Krull KR, Brinkman TM, Li C, et al: Neurocognitive Outcomes Decades After Treatment for Childhood Acute Lymphoblastic Leukemia: A Report From the St. Jude Lifetime Cohort Study. J Clin Oncol, 2013
4. Conklin HM, Krull KR, Reddick WE, et al: Cognitive outcomes following contemporary treatment without cranial irradiation for childhood acute lymphoblastic leukemia. J Natl Cancer Inst 104:1386-95, 2012
5. Jain N, Brouwers P, Okcu MF, et al: Sex-specific attention problems in long-term survivors of pediatric acute lymphoblastic leukemia. Cancer 115:4238-45, 2009
6. Reddick WE, Taghipour DJ, Glass JO, et al: Prognostic factors that increase the risk for reduced white matter volumes and deficits in attention and learning for survivors of childhood cancers. Pediatr Blood Cancer 61:1074-9, 2014
7. Kimura D: Sex, sexual orientation and sex hormones influence human cognitive function. Curr Opin Neurobiol:259-63, 1996
Figures
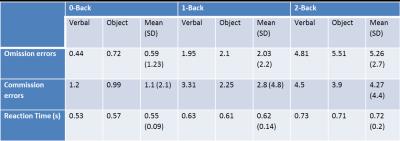
Table 1: N-back performance during fMRI in a population of ALL survivors.
Note: Omission error: failing to respond when appropriate; Commission error: responding when not appropriate; Reaction time: time necessary to respond when appropriate.