4598
Hope and the brain: trait hope mediates the protective role of the medial orbitofrontal cortex spontaneous activity against anxiety1Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu, People's Republic of China, 2Department of Psychiatry, West China Hospital of Sichuan University, Chengdu, People's Republic of China
Synopsis
As a personality trait, hope refers to the motivational tendency for initiating actions and generating routes to achieving goals and plays a protective role in anxiety. Here, we investigated the neural basis of hope in 231 adolescents using resting-state functional magnetic resonance imaging (RS-fMRI). We found that trait hope was negatively associated with the spontaneous activity in the bilateral medial orbitofrontal cortex (mOFC). Further mediation analyses revealed that trait hope mediated the relationship between the mOFC activity and trait anxiety. Taken Together, our findings might provide the initial evidence for the brain-personality mechanisms protecting against anxiety.
Purpose
Hope is a goal-oriented expectation which involves both the motivation to carry out tasks (agency) and the cognitive ability to find ways to achieve goals (pathways)1. As many studies have shown, hope is found to be linked with mental health, happiness and achievement. Specially, hope plays a protective role in anxiety symptoms among both healthy and clinical participants and draws increasing attention as a recovery factor to resist anxiety 2. However, the neural mechanism of hope remains largely unknown. In this study, we explored the neural correlates of trait hope and their relation to anxiety in a large sample of adolescent students via fractional amplitude of low-frequency fluctuations (fALFF) using resting-state functional magnetic resonance imaging (RS-fMRI).Methods
The participants include 231 healthy adolescent students (age = 18.48 ± 0.54 years, 121 females) at several local public high schools in Chengdu, China. We used the Dispositional Hope Scale (DHS) to measure the individual differences in trait hope and used the State-Trait Anxiety Inventory-Trait (STAI-T) to measure anxiety levels of the individuals. In addition, we used the Positive and Negative Affect Schedule (PANAS) to rule out the possible influence of the positive and negative affect on trait hope and spontaneous brain activity. The RS-fMRI data was collected using a 3.0 T Siemens-Trio Erlangen MRI scanner with a 12-channel phase array head coil. An echo-planar imaging (EPI) sequence was employed to obtain the data: 240 volumes; TR/TE, 2000/30 ms; field of view, 240 mm × 240 mm; flip angle, 90°; matrix, 64 × 64; voxel size, 3.75 × 3.75 × 5 mm3; slice thickness, 5 mm; interslice gap, 0 mm; 30 slices. During scanning, the subjects were asked to stay awake with eyes closed and to not think about anything particularly. We used the DPARSF software to preprocess the imaging data and the main steps included the analyses of slice timing and head movement correction, normalization, smoothing and linear trends removing. Then, the fALFF calculation was conducted. To identify the brain regions of trait hope, we performed a whole-brain correlation analysis between the fALFF in each voxel and DHS score. The significant cluster was set at p < 0.05 at cluster level with 10,000 iterations, combining with p < 0.005 at single voxle level (cluster size ≥ 50 voxels, 1350 mm3). Next, we employed prediction analyses with machine learning approach to investigate the predictive ability of brain activity on trait hope. Finally, we conducted mediation analyses to confirm whether trait hope can mediate the association between brain activity and anxiety.Results
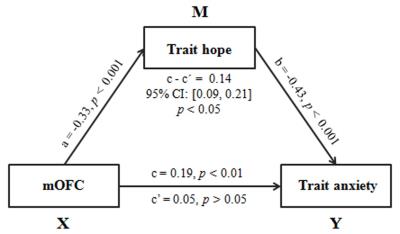
The whole-brain correlation analyses revealed that higher trait hope was related to lower fALFF in the bilateral medial orbitofrontal cortex (mOFC; MNI coordinates: 2,64,-12; cluster size = 2727 voxels; r = -0.33, p < 0.001; Figure 1) after controlling for age and gender. The prediction analyses showed that, after adjusting for age and gender, the fALFF in the mOFC can reliably predict individual differences in trait hope [r (predicted, observed) = -0.26, p < 0.001]. Furthermore, mediation analyses suggested that trait hope played a mediating role in the association between the fALFF in the mOFC and trait anxiety (indirect effect =, 95% CI = [0.09, 0.21 ], p < 0.05; Figure 2). Finally, our results persisted even after adjusting for the effect of positive and negative affect, indicating the specificity of these effects.Discussion
The present study examined the neural substrates of trait hope and the role of trait hope in the relationship between spontaneous brain activity and anxiety. First, we found a negative association of trait hope with the fALFF in the mOFC, which was consistent with previous investigations revealing a higher activity of mOFC in patients with psychiatric disorders, such as social anxiety disorder 3 and obsessive–compulsive disorder 4. Moreover, previous studies have indicated that the activity of the mOFC is linked with motivation producing 5, development of goal-directed behaviors and approach-oriented coping strategies 6, which may determine the related components of trait hope. Second, this research revealed that trait hope mediated the influence of the fALFF in the mOFC on trait anxiety. This result indicates that trait hope is likely a positive factor in protecting the anxiety symptoms of individuals and highlights that trait hope might act as an underlying mechanism for explaining the common variance between spontaneous brain activity and trait anxiety.Conclusion
In conclusion, our research sheds light on the underlying brain basis that determines individual differences in trait hope and reveals a potential brain-personality mechanism for protecting against anxiety symptoms.Acknowledgements
This study was funded by the National Natural Science Foundation (grants 81222018, 81371527, 81030027, 81227002, 81401398,and 81220108013), the National Key Technologies R&D Program (program 2012BAI01B03), and the Program for Changjiang Scholars and Innovative Research Team in University (grant IRT1272) of China. The authors declare no competing interests.References
1. Snyder C R, Harris C, Anderson J R, et al. The will and the ways: development and validation of an individual-differences measure of hope[J]. Journal of personality and social psychology, 1991, 60(4): 570.
2. Arnau, R. C., Rosen, D. H., Finch, J. F., Rhudy, J. L. & Fortunato, V. J. Longitudinal effects of hope on depression and anxiety: A latent variable analysis. Journal of Personality,2007; 75, 43-63.
3. Bruhl, A. B., Delsignore, A., Komossa, K. & Weidt, S. Neuroimaging in social anxiety disorder-a meta-analytic review resulting in a new neurofunctional model. Neurosci Biobehav Rev, 2014; 47, 260-80.
4. Hou, J. M., Wu, W. J., Lin, Y., Wang, J., Zhou, D. Q., Guo, J. W., Gu, S. S., He, M., Ahmed, S., Hu, J. N., Qu, W. & Li, H. T. (2012). Localization of cerebral functional deficits in patients with obsessive-compulsive disorder: A resting-state fMRI study. Journal of Affective Disorders, 2012; 138, 313-321.
5. Linke, J., Kirsch, P., King, A. V., Gass, A., Hennerici, M. G., Bongers, A. & Wessa, M. Motivational orientation modulates the neural response to reward. Neuroimage, 2010; 49, 2618-25.
6. Eddington, K. M., Dolcos, F., Cabeza, R., Krishnan, K. R. R. & Strauman, T. J. Neural correlates of promotion and prevention goal activation: An fMRI study using an idiographic approach. Journal of Cognitive Neuroscience, 2007; 19, 1152-1162.
Figures