4596
Evidence of a link between brain structure and function and gut permeability: a combined RS-fMRI and DTI investigation of the brain-gut axis in healthy women1CMIV, Linöping University, Linköping, Sweden, 2Dept. of Clinical and Experimental Medicine, Linköping University, 3Institute of Medical Psychology & Behavioral Immunobiology, University of Duisburg-Essen, Essen, Germany, 4Dept. of Medical and Health Sciences, Linköping University, 5Dept. of Medicine, UCLA, Los Angeles, CA, United States
Synopsis
The brain-gut axis is thought to play a key role in the regulation of the gastrointestinal system with overall physical and emotional health. In diseases, such as irritable bowel syndrome, dysfunction within brain-gut interactions have been proposed to underlie symptoms of chronic abdominal pain. This study demonstrated that variations in gut mucosal permeability affected both resting-state functional connectivity in the DMN and white matter microstructure properties in healthy adult women. Variations within brain function and structure were apparent even when variations in gut permeability were small and remained within the normal range, indicating that brain-gut interactions may be quite sensitive.
INTRODUCTION
The brain-gut axis is thought to play a key role in the regulation
of the gastrointestinal system with overall physical and emotional health. In diseases, such as irritable bowel
syndrome, dysfunction within brain-gut interactions have been proposed to
underlie symptoms of chronic abdominal pain.1 Changes in gut mucosal permeability have been
implicated in sensitizing pain pathways.2 Similarly, changes in resting-state
functional connectivity, especially in the default mode network, have been
shown to change in patients reporting chronic pain.3 To date, though, no study has investigated
brain-gut interactions in either healthy or diseased populations using organic
measures of both gut and brain structure and function. The aim of the current study was to examine
the relationships between in vitro measures of gut mucosal permeability and
resting state functional connectivity in the default mode network and whole
brain white matter microstructure in a population of healthy women. We anticipated observing that increases in
gut mucosal permeability would be related to increased functional and
structural connectivity among brain regions responsible for processing pain
signals in the brain.
METHODS
Fifteen healthy women were included in the
study (mean age = 29.7 yrs). Ten minutes
of eyes closed resting state fMRI data (TR/TE = 2000/37ms; voxel =
3.59x3.59x4mm3; 28 slices; 300 time points) and diffusion weighted
MRI data (TR/TE = 9339/82ms; voxel = 2mm3; 64 directions; b = 1000)
were acquired on a 3T scanner (Philips Ingenia). FMRI data were preprocessed (realignment, normalization
to MNI space, smoothing with 8mm FWHM Gaussian kernel) in SPM8. The GIFT group independent component analysis
toolbox was used to identify the default mode network (DMN). The diffusion data were processed using
standard procedures in FSL, and group-level maps of fractional anisotropy (FA)
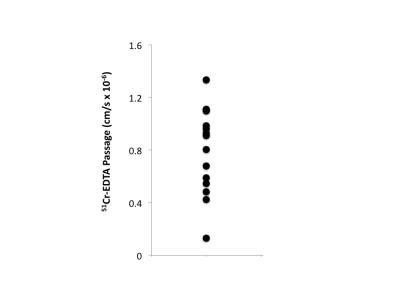
were calculated using tract based spatial statistics (TBSS). Colonic biopsies were acquired within two
weeks post-MRI scanning after 8 hours of fasting. Biopsy samples were mounted in Ussing
chambers4,5, and a paracellular probe (51chromium
(Cr)-EDTA) was added to the mucosal side of the chambers. Passage of 51Cr-EDTA through the
biopsies to the serosal side of the chambers was measured by gamma counting
after 60 and 120 minutes (Figure 1). Whole brain covariate analyses were
performed in PALM6 for the DMN and FA data separately to determine
the relationship between paracellular passage of 51Cr-EDTA and DMN
functional connectivity and FA values, respectively.
RESULTS
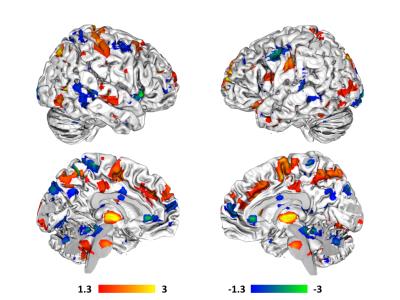
Variations in gut mucosal paracellular permeability correlated
with significant whole-brain variations in resting state functional
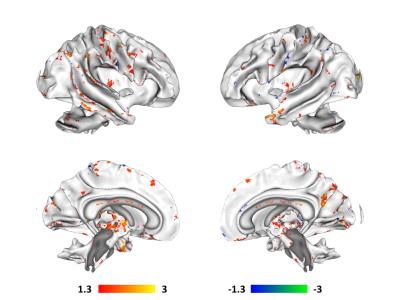
connectivity in the DMN (Figure 2) and white matter microstructure as measured
by FA (Figure 3). Increases in paracellular
permeability correlated with increased connectivity between the DMN and a
number of regions thought to comprise the ascending and descending pain
pathways in the brain, including thalamus, supplementary motor area, right
middle frontal gyrus, raphe nucleus magnus, and bilateral post-central
gyri. Decreases in paracellular
permeability also correlated with increased connectivity between DMN and pain
processing related brain regions, including ACC, right insula, bilateral
precentral gyri, and periaqueductal grey.
For white matter microstructure, increases in paracellular permeability
in the gut correlated with increased FA in a number of major tracts, including
corticospinal, corticothalamic, anterior thalamic radiations, corpus callosum,
superior longitudinal fasciculi, as well as a number of association
fibers. Decreases in paracellular
permeability only appeared to correlate predominantly with increased FA in
association fibers.
CONCLUSIONS
This study has demonstrated that variations in gut mucosal permeability may affect both resting-state functional connectivity in the DMN and white matter microstructure properties in healthy adult women. In the case of increases in paracellular permeability, the results indicate good anatomical correspondence between the white matter tracts exhibiting increased FA and the brain regions exhibiting increased functional connectivity, suggesting a structure-function match. The results also indicate that gut mucosal paracellular permeability is processed as pain. The results further suggest that variations within the brain are apparent even when variations in gut permeability are small and remain within the range for gut mucosal paracellular permeability previously reported in healthy adults5, indicating that brain-gut interactions may be quite sensitive. It is, however, still unclear whether these variations are temporary and/or modifiable, or whether variations in gut permeability precede variations in brain function and structure or vice versa.Acknowledgements
No acknowledgement found.References
1Mayer EA and Tillisch K, Annual Review of Medicine, 2011; 6:381-96.
2Vergnolle N et al., Nature Medicine, 2001; 7: 821-6.
3Farmer MA et al., Neuroscience Letters, 2012; 520: 197-203.
4Grass GM et al., Pharmacological Research, 1988; 5: 372-6.
5Keita AV et al., Laboratory investigation: a journal of technical methods and pathology, 2010; 59: 1213-21.
6Winkler AM et al., NeuroImage, 2014; 92: 381-97.
Figures