4595
Altered resting state functional connectivity by autogenic training1Radiology, Nihon University School of Medicine, Tokyo, Japan
Synopsis
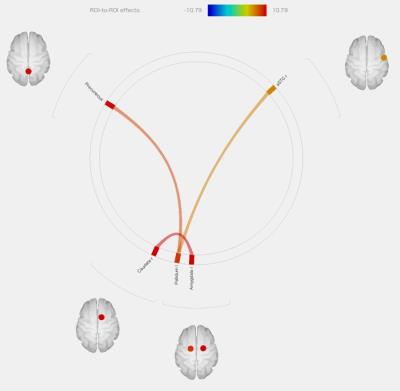
Objective: We investigated whether autogenic training (AT) altered resting state functional connectivity. Methods: 7 volunteers familiar with AT participated in this study. Rs-fMRI was performed pre and post AT, and 3D-T1WI was acquired. Group analysis was performed to explore the alteration of resting functional connectivity after autogenic training by using CONN functional connectivity toolbox. Results: Greater connectivity between 1. right amygdala and right caudate, 2. precuneus and left pallidum, and 3. right supratemporal gyrus and left pallidum, were demonstrated. Conclusion: AT could have positive effects not only to restore the automatic nervous system but also to alter RS functional connectivity.
PURPOSE
Autogenic training (AT) is a common and clinically used auto-hypnotic relaxation technique. It specifically aims at stress prevention, and also AT supposedly has a positive effect such as tension headache/migraine, chronic pain syndrome, anxiety disorders1-2. Resting-state functional magnetic resonance imaging constitutes a novel paradigm that examines spontaneous brain function by using blood oxygen level - dependent contrast in the absence of a task. Alterations in resting state networks (RSNs) have been identified in the above-mentioned diseases3-4. We investigated whether RSN altered by AT.METHODS
3 females and 4 male healthy volunteers familiar with AT (5 psychosomatic doctors and 2 clinical psychotherapists) participated in this study. MRI scans were acquired with a GE discovery 750W 3.0 T scanner equipped with 32 channel head coils. Firstly, resting-state echo-planar imaging (EPI) scan (38 axial slices, slice thickness=3.8mm, TR=2000ms, TE=30ms, matrix 64x64, FOV 23cm, 200 volumes, 6min40s) was acquired. Subsequently, participants conducted autogenic training for 5 minutes, and resting-state EPI scan was acquired again. Finally, 3D brain volume sequence (BRAVO) T1-weighted anatomical images (200 contiguous sagittal slices) were also acquired (TR = 8.5ms; TE = 3.2ms; flip angle 12°; matrix 256 × 256; FOV 25.6 cm, 5min). The data were pre-processed and analyzed using SPM12 (The Welcome Department of Cognitive Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm/software/spm12), and functional connectivity analysis was carried out using the CONN-fMRI Functional Connectivity toolbox v15a (http://www.nitrc.org/projects/conn). Predefined regions-of-interest from the CONN-fMRI Functional Connectivity toolbox were chosen as seeds to create connectivity maps, and group analysis was performed to explore the alteration of resting functional connectivity after autogenic training. Significance level was set at P value of no more than 0.05 with family-wise error (FWE) correction for multiple comparisons.RESULTS
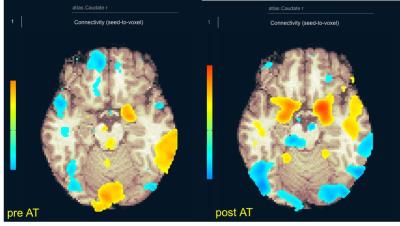
Greater connectivity between 1. right amygdala (limbic system) and right caudate (P=0.0051), 2. precuneus (DMN) and left pallidum (P=0.0173), and 3. right supratemporal gyrus (auditory system) and left pallidum (P=0.0428), were demonstrated (figure1). Figure 2 shows an individual resting functional MR image before and after AT (seed: right caudate). Increased functional connectivity in amygdala was demonstrated.DISCUSSION
We used RS functional MR imaging to investigate the alteration of the functional connectivity after AT. Increased RS functional connectivity of the DMN, limbic region, basal ganglia, and auditory system, known as resting state functional networks, were demonstrated. The DMN was implicated in self-referential and reflective activity, including episodic memory retrieval, emotions, and planning future events5. Previous neuroimaging studies reported that DMN abnormalities are associated psychiatric disorders, which may underlie cognitive and affective processing problems6. The limbic system is the central part of the so-called emotional brain circuitry, dedicated to the processing and regulation of emotion. Previous RS functional MR imaging studies reported the alteration frontal lobe and/or limbic structures in mood disorders6. Anterior / posterior cingulate cortex, prefrontal cortex including the DMN, and insula and amygdala including limbic lesion form pain modulatory network that might be involve in psychological modulation of pain7. AT has been engaged in restoring the balance between activity of the sympathetic and parasympathetic branches of the autonomic nervous system, which can be modulated to a more relaxed state1. AT could have such positive effects not only to restore the automatic nervous system but also to lessen symptomatic states by altering RS functional connectivity. It could be also for the same reason that coffee, nicotine, and food intake influencing the autonomic nervous system were reported as confounding factors of RS functional connectivity5.CONCLUSION
AT could have such positive effects not only to restore the automatic nervous system but also to lessen symptomatic states by altering RS functional connectivity.Acknowledgements
No acknowledgement found.References
1 Jensen M.P. and D.R. Patterson. Hypnotic approaches for chronic pain management: clinical implications of recent research findings. Am Psychol 69(2): 167-177, 2014.
2 Golding K., et al. Self-help relaxation for post-stroke anxiety: a randomised, controlled pilot study. Clin Rehabil 30(2): 174-180, 2016.
3 Otti A., et al. Functional network connectivity of pain-related resting state networks in somatoform pain disorder: an exploratory fMRI study. J Psychiatry Neurosci 38(1): 57-65, 2013.
4 Toazza R., et al. Amygdala-based intrinsic functional connectivity and anxiety disorders in adolescents and young adults. Psychiatry Res 257: 11-16, 2016.
5 Barkhof F., et al. Resting-state functional MR imaging: a new window to the brain. Radiology 272(1): 29-49, 2014.
6 Akansha B.A., et al. The Significance of the Default Mode Network (DMN) in Neurological and Neuropsychiatric Disorders: A Review. Yale J Biol Med. 24;89(1):49-57, 2016.
7 Khan, S.A., et al. Altered structure and function in the hippocampus and medial prefrontal cortex in patients with burning mouth syndrome. Pain 155(8): 1472-1480, 2014.
Figures