4586
Abnormal resting-sate functional connectivity of thalamus in patients with paroxysmal kinesigenic dyskinesia1Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital, Sichuan University, Chengdu, People's Republic of China, 2Department of Neurology, West China Hospital, Sichuan University, Chengdu, People's Republic of China
Synopsis
The underlying pathophysiological mechanisms of paroxysmal kinesigenic dyskinesia are still not fully understood. The aim of this study was to investigate the patterns of resting-sate functional connectivity of thalamus in patients with paroxysmal kinesigenic dyskinesia. Our novel findings of abnormal resting-sate functional connectivity from thalamus to precentral gyrus and medial frontal gyrus provided direct evidence that the basal ganglia-thalamo-cortical circuit may play an important role in the pathogenesis of paroxysmal kinesigenic dyskinesia.
Purpose
To investigate the patterns of functional connectivity of thalamus in patients with paroxysmal kinesigenic dyskinesia using resting-sate functional MRI.Methods
This study was approved by the local ethical committee, and written informed consent was obtained from all participants. Patients with paroxysmal kinesigenic dyskinesia, along with healthy control subjects, were recruited from the West China Hospital, Sichuan University. Diagnosis was made independently by two neurologists based on the criteria proposed by Bruno M. K. et al1.
MR imaging data were collected using a 3-T Siemens Trio Tim system with a 12-channel phased-array head coil. Resting-state functional images were acquired axially with an echo-planer imaging (EPI) sequence (30 slices, 205 volumes, TR=2000 ms, TE = 30 ms, FOV = 24 cm, matrix = 64 × 64, and a flip angle = 90). Image preprocessing was performed by the Data Processing Assistant for Resting-State fMRI (DPARSF, http://www.restfmri.net)2 and Statistical Parametric Mapping (SPM8, Wellcome Trust Centre for NeuroImaging, UCL, UK). Processing steps included removing the first 10 images, slice time correction, realign, spatial normalization, spatial smoothing (8mm full width at half maximum), linear trend removal, temporal band-pass filtering (0.01–0.08 Hz) and regressing out the nuisance signals. We defined respectively the bilateral thalami as seed regions (MNI coordinate: x = 6, y = -16, z = 4; x = -6, y = -16, z = 4; 6mm in radius) to compute the functional connectivity according to the previous studies3,4. In addition, we extracted the functional connectivity strengths of these significant regions identified from the patient group and performed the correlation analysis with illness duration.
The two-sample t-test was used for the group comparisons between the patients with paroxysmal kinesigenic dyskinesia and control subjects. The result was corrected with AlphaSim program, which setting the voxel-wise threshold at P < 0.01 and cluster size > 71 voxels determined by Monte Carlo simulation (1000 iterations), corresponding to a corrected P < 0.05. Pearson’s correlation was calculated with illness duration within the patients group using SPSS 20.0 software.
Results
A total of 60 patients with paroxysmal kinesigenic dyskinesia (mean age ± SD:23.0±7.8 years) and 62 healthy control subjects (mean age ± SD:23.4±4.7 years) were included in the present study. No significant differences were detected between the two groups in age (P=0.73) and gender (P=0.17).
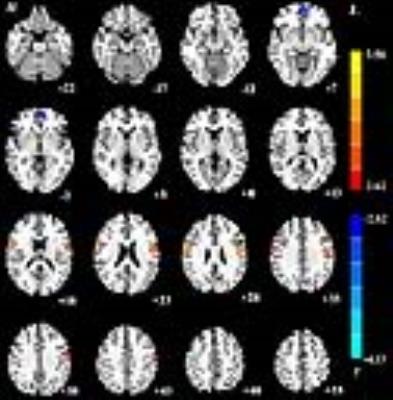
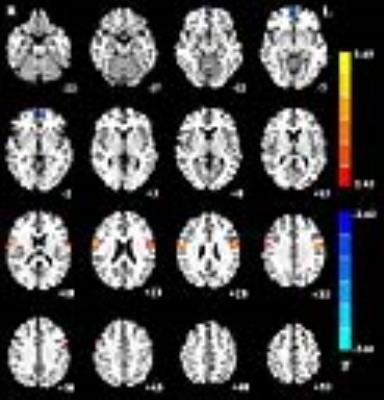
The right thalamus showed significantly increased functional connectivities to the bilateral precentral gyri (x=60, y=-3, z=24, cluster=160; x=-48, y=-3, z=27, cluster=183) and decreased functional connectivity to the left medial frontal gyrus (x=-6, y=57, z=-6, cluster=73) between patients with paroxysmal kinesigenic dyskinesia and healthy controls (Figure 1). The left thalamus showed significantly increased functional connectivities to the bilateral precentral gyri (x=60, y=-3, z=27, cluster=122; x=-51, y=-6, z=33, cluster=162) and decreased functional connectivity to the left medial frontal gyrus (x=-6, y=60, z=-6, cluster=82) between patients with paroxysmal kinesigenic dyskinesia and healthy controls (Figure 2). Correlation analyses showed no significant correlations between the functional connectivity strengths from the bilateral thalami to these brain regions identified and illness duration.
Discussion
Paroxysmal kinesigenic dyskinesia is a rare neurological disorder, characterized by sudden, brief attacks of involuntary dyskinetic movements that are exclusively triggered by initiation of voluntary movements5. The underlying pathophysiological mechanisms are still not fully understood. Previous studies reported the increase fractional anisotropy in thalamus using diffusion tensor imaging3,4. In our study, we defined the bilateral thalami as seed regions to investigate the patterns of resting-sate functional connectivity of thalamus in patients with paroxysmal kinesigenic dyskinesia.
Our results revealed that the functional connectivity between the thalamus and motor cortex was significantly increased, which may be associated with involuntary dyskinetic movements of this disease. In addition, our study also found decreased functional connectivity between the thalamus and medial frontal gyrus, which may suggest the impairment of normal inhibition against competing motor.
Conclusion
Our novel findings of abnormal resting-sate functional connectivity from thalamus to precentral gyrus and medial frontal gyrus provided direct evidence that the basal ganglia-thalamo-cortical circuit may play an important role in the pathogenesis of paroxysmal kinesigenic dyskinesia.Acknowledgements
No acknowledgementsReferences
1. Bruno MK, Hallett M, Gwinn-Hardy K, et al. Clinical evaluation of idiopathic paroxysmal kinesigenic dyskinesia: new diagnostic criteria. Neurology. 2004;63(12):2280-2287.
2. Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB Toolbox for "Pipeline" Data Analysis of Resting-State fMRI. Frontiers in systems neuroscience. 2010;4:13.
3. Kim JH, Kim DW, Kim JB, Suh SI, Koh SB. Thalamic involvement in paroxysmal kinesigenic dyskinesia: a combined structural and diffusion tensor MRI analysis. Human brain mapping. 2015;36(4):1429-1441.
4. Zhou B, Chen Q, Gong Q, Tang H, Zhou D. The thalamic ultrastructural abnormalities in paroxysmal kinesigenic choreoathetosis: a diffusion tensor imaging study. Journal of neurology. 2010;257(3):405-409.
5. Bhatia KP. The paroxysmal dyskinesias. Journal of neurology. 1999;246(3):149-155.
Figures