4584
NODDI revealed the brain microstructural damage in patients with moyamoya disease1Department of Radiology, Juntendo University, Tokyo, Japan, 2Department of Neurosurgery, Tokyo Medical and Dental University, Tokyo, Japan
Synopsis
We applied Neurite Orientation Dispersion and Density Imaging (NODDI) and neurophysiological batteries to 13 patients with moyamoya disease (10 females, age 16-61 yo). We found that intracellular volume fraction (Vic) and orientation dispersion index (OD) decreased as the stages of vascular lesion progressed, and many neurocognitive tasks correlated with the decrease in Vic and OD among different parts of brain. Interestingly, some tasks were much more correlated with the Vic and OD among posterior part of brain than among frontal part. This finding may suggest the importance of PCA lesions in neurocognitive disturbance in patients with moyamoya disease.
PURPOSE
Moyamoya disease is a slowly progressive cerebrovascular disease which causes not only stroke, but also neurocognitive dysfunction in young adults. To investigate the brain microstructural change provoked by chronic ischemia and its correlation with neurocognitive dysfunction, we applied Neurite Orientation Dispersion and Density Imaging (NODDI)1 to patients with moyamoya disease.METHODS
This prospective study was approved by ethical committee of two institutes. From Dec 2015 to Sep 2016, 20 patients with moyamoya disease were enrolled in this study. In the present analysis, we excluded 7 patients with large cortical infarctions and analyzed 13 patients (10 females, age 16-61 yo, 9-22 years of schooling, including 1 patient with hemorrhage and 1 postoperative patient). ICA and PCA staging2 (Stage 1 to 4) of all patients were assessed by MRA. All patients underwent neurophysiological batteries including WAIS-III, TMT and personality test NEO-FFI. NODDI data as well as 3-dimensional T1-weighted imaging was acquired using a 3.0-T MRI system (Magnetom Skyra; Siemens AG, Erlangen, Germany) with 3 b values (0, 700: 30 MPG axes, 2850: 60 MPG axes) and then fitted to the NODDI model by using the NODDI Matlab Toolbox1. Created maps of orientation dispersion index (OD) and intracellular volume fraction (Vic) were fitted to standard template via 3DT1 by SPM12, and the white matter lesions and hemorrhage visible on conventional MRI and b=0 images were deleted from each map by drawing volume of interests using MRIcron. After the smoothing of each map, multiple regression analysis was performed by SPM 12 to reveal the regions with significant corelation with ICA and PCA staging score and neuropsychological performance. Age, sex and years of schooling were included as covariates. The initial voxel threshold was set to .001 uncorrected and clusters were considered as significant when falling below a cluster-corrected p(FEW)=.05.RESULTS
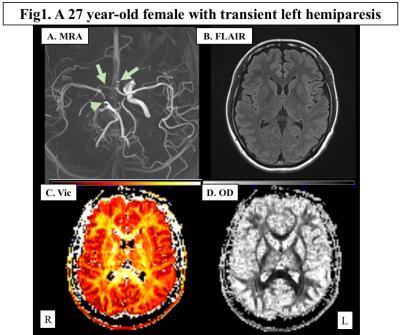
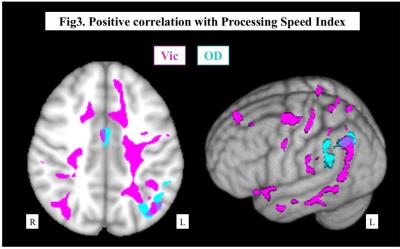
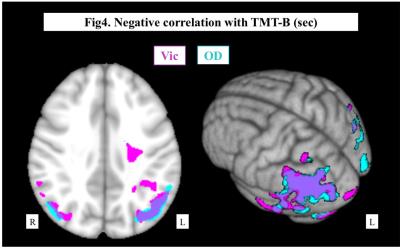
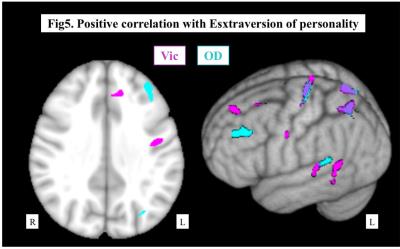
Representative maps were shown in Fig.1. Both Vic and OD of the cerebral hemisphere decreased as the PCA and ICA stages progressed, and the PCA stage had significant correlation with Vic and OD in more widespread areas than ICA stage (Fig.2). In regard of neurocognitive performance, each task score had significant correlation with Vic and OD at different areas of the brain. As shown in Fig.3, Processing Speed Index of WAIS-III was positively correlated with Vic in the widespread left hemisphere, mainly in the deep white matter. On the other hand, significant correlation with TMT-B was mainly seen in the bilateral parietotemporal areas, predominantly in the left side. The degree of Extraversion in the personality test correlated with Vic and OD at multiple regions in the left frontal, parietal and temporal lobes.DISCUSSION
In this study, Vic and OD decreased as the vascular lesion progressed. The decrease in Vic and OD must reflect the decreased neurites and networks created by axons and dendrites due to chronic ischemia. We found that the PCA stage was more strongly correlated with the decrease in Vic and OD in the cerebral hemisphere than the ICA stage. We considered that this finding was related to the nonpararell involvement of ICA and PCA in moyamoya disease2. All patients must at least have some degree of ICA lesions as that is the diagnostic criteria of moyamoya disease. On the other hand, the degree of PCA stages widely varies among patients. The intact PCAs might have an important role to compensate the hemodynamic stress caused by lesions in anterior circulation, and, therefore, appearance of PCA lesion and its progress must heavily influence on the degree of neuronal damage.
We found some task performance was more correlated to the areas of posterior circulation than in the frontal areas, where diffusion parameters were reported to be correlated with neurophysiological performance of moyamoya disease3,4. We speculated that this result could be explained hy the significance of PCA lesions as presented above. Several previous papers suggested the degree of PCA lesions coupled with poorer prognosis, more infarction and lower IQ5,6. Another paper reported cerebral oxygen the metabolic in posterior temporal lobe and occipital lobe correlated with cognitive performance as well as of frontal areas in PET study7. Our present result on the correlation between PCA stage and Vic and OD may also suggest the neuronal damage of posterior part of brain may play significant role in the decline of neurocognitive function in patients with moyamoya disease.
CONCLUSION
NODDI is a promising tool to evaluate brain microstructural change and neurocognitive dysfunction in patients with moyamoya disease. Further enrollment of patients and analysis will be conducted.Acknowledgements
No acknowledgement found.References
1. Zhang, H., Schneider, T., Wheeler-Kingshott, C. A. et al. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage, 2012; 61, 1000-1016.
2. Mugikura, S., Takahashi, S., Higano, S., et al. Predominant Involvement of Ipsilateral Anterior and Posterior Circulations in Moyamoya Disease. Stroke, 2002; 33, 1497-1500.
3. Kazumata, K., Tha, K. K., Narita, H., et al. Chronic ischemia alters brain microstructural integrity and cognitive performance in adult moyamoya disease. Stroke, 2015; 46, 354-360.
4. Kazumata, K., Tha, K. K., Narita, H., et al. Characteristics of Diffusional Kurtosis in Chronic Ischemia of Adult Moyamoya Disease: Comparing Diffusional Kurtosis and Diffusion Tensor Imaging. American Journal of Neuroradiology, 2016; Epub ahead of print.
5. Miyatake, S., Miyake, N., Touho, H., et al. Homozygous c.14576G>A variant of RNF213 predicts early-onset and severe form of moyamoya disease. Neurology, 2012; 78(11), 803-810.
6. Mukawa, M., Nariai, T., Matsushima, Y., et al. Clinical features of familial juvenile cases of moyamoya disease: analysis of patients treated in a single institute over a 28-year period. J Neurosurg Pediatr, 2013; 12(2), 175-180.
7. Hosoda, C., Nariai, T., Ishiwata, K., et al. Correlation between focal brain metabolism and higher brain function in patients with Moyamoya disease. Int J Stroke, 2010; 5(5), 367-373.
Figures