4582
Visualization of Post Stroke Revascularization on a Rat Model Using Dual R1- and Compressed Sensing Assisted R2*-MRAs1Department of Biomedical Engineering, Ulsan National Institute of Science and Technology, Ulsan, Korea, Republic of
Synopsis
The visualization of post stroke revascularization is of particular importance for the prognosis and therapeutic measures. In this work, using dual contrast R1-R2*-MRAs, visualization of post stroke revascularization on a rat model was performed. R1-R2*-MRAs were used to visualize surface and inner regions of rat brain, respectively. As a result of post stroke revascularization, all MRAs clearly showed thickened vessel structures in ipsilateral hemisphere.
Introduction
Among stroke, which is the fifth leading cause of death in the US1, the ischemic stroke is most common. Ischemic stroke occurs when insufficient blood is supplied to the brain, and transient middle cerebral artery occlusion (MCAO) model is widely used as a corresponding model. The visualization of post stroke revascularization2-4 is of particular importance for the prognosis and therapeutic measures. In this work, highly resolved (59 μm3 isotropic) dual contrast MRAs acquisition was applied on a rat transient MCAO model to visualize post-ischemic brain vasculature5,6. By using superparamagnetic iron-oxide nanoparticles (SPION) as a single contrast agent, dual contrast R1-R2*-MRAs were sequentially acquired. R1-R2*-MRAs were used to visualize post stroke revascularization of surface and inner regions of rat brain, respectively. Also, compressed sensing (CS) technique was applied to R2*-MRA to further reduce scan time7.Methods
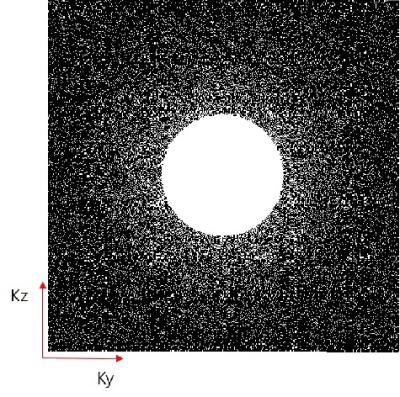
Normal Wistar rat (6 weeks, 184 g) underwent one hour of MCAO and 72 hours of reperfusion. Dual contrast MRAs of rat were acquired with a 7 T MR scanner (Bruker, Ettlingen, Germany). For R1- R2*-MRAs, Ultrashort echo time (UTE) and Fast low angle shot (FLASH) sequences were used, respectively. All MRAs were acquired after injection of SPION at a dose of 240 μmol Fe/kg. Imaging parameters for UTE sequence were as follows: flip angle (FA) = 40°; TR = 22 ms; TE = 0.0256 ms; field of view (FOV) = 30 × 30 × 30 mm3; matrix size = 512 × 512 × 512; resolution = 59 × 59 × 59 μm3. Imaging parameters for FLASH sequence were as follows: FA = 20°; TR = 320 ms; TE = 12 ms; FOV = 30 × 30 × 30 mm3; matrix size = 512 × 512 × 512; resolution = 59 × 59 × 59 μm3; CS acceleration factor = 4. Figure 1 shows 3-dimensional k-space undersampling scheme for FLASH sequence. As frequency encoding is free, frequency encoding steps are fully sampled. The center region of k-space was always sampled, because the center region has the highest signal intensity. The remaining regions were sampled following Gaussian distribution. CS reconstruction was achieved by solving the constrained optimization problem7.Results
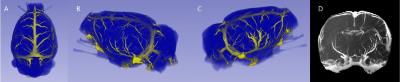
One dorsal and two lateral views of rat brain surfaces from R1-MRAs are shown in Figure 2. A, B and C. As a result of post stroke revascularization, two lateral views show different vascular structures. Thickened and twisted arteries are apparent on the cortical surface of ipsilateral hemisphere, in comparison with those of contralateral hemisphere. Anterior-to-posterior maximum intensity projection (MIP) view of R1-MRA is shown in D. Ipsilateral hemisphere is populated with many cortical vessels, which are unobservable in contralateral hemisphere. In Figure 3, minimum intensity projection (MinIP) views of R2*-MRAs are shown. The slice thickness are 0.885 mm for dorsal and lateral slices and 0.59 mm for anterior-to-posterior slices as shown in Figure 3A-3E. Thickened vessel structures and hypointense regions are apparent in ipsilateral hemisphere.Discussion
In this study, dual contrast MRAs using single SPION contrast agent was performed on a rat transient MCAO model to highly resolve post ischemic revascularizations of entire brain. R1-MRA provided accurate vessel structures in the brain surface region. R2*-MRA was especially sensitive to intracortical penetrating vessels. Both R1-R2*-MRAs clearly showed thickened vessel structures in ipsilateral hemisphere. Further study is required for the high-resolution longitudinal monitoring of revascularization process including apparent surface and inner cortical vessel thickenings.Acknowledgements
No acknowledgement found.References
1. Kochanek, Kenneth D., et al. "Deaths: final data for 2014." National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System 65.4 (2016): 1.
2. Lin, Chien-Yuan, et al. "Dynamic changes in vascular permeability, cerebral blood volume, vascular density, and size after transient focal cerebral ischemia in rats: evaluation with contrast-enhanced magnetic resonance imaging." Journal of Cerebral Blood Flow & Metabolism 28.8 (2008): 1491-1501.
3. Lin, Chien-Yuan, et al. "In vivo cerebromicrovasculatural visualization using 3D ΔR 2-based microscopy of magnetic resonance angiography (3DΔR 2-mMRA)." Neuroimage 45.3 (2009): 824-831.
4. Huang, Chien-Hsiang, et al. "High-resolution structural and functional assessments of cerebral microvasculature using 3D gas ΔR 2*-mMRA." PloS one 8.11 (2013): e78186.
5. Jung, Hoesu, et al. "Dual MRI T 1 and T 2 (*) contrast with size-controlled iron oxide nanoparticles." Nanomedicine: Nanotechnology, Biology and Medicine10.8 (2014): 1679-1689.
6. Jung, H. S., et al. "UTE–ΔR2–ΔR2* combined MR whole-brain angiogram using dual-contrast superparamagnetic iron oxide nanoparticles." NMR in Biomedicine 29.6 (2016): 690-701.
7. Lustig, Michael, David Donoho, and John M. Pauly. "Sparse MRI: The application of compressed sensing for rapid MR imaging." Magnetic resonance in medicine 58.6 (2007): 1182-1195.