4579
Water Diffusion Heterogeneity in Cytoxic EdemaKevin Midlash1, Yong Jeong2, Charles Cantrell2, Keigo Kawaji1, Greg Christoforidis1, and Timothy J. Carroll1
1University of Chicago, Chicago, IL, United States, 2Northwestern, Evanston, IL, United States
Synopsis
Modern stroke research and treatment depend on the ability to accurately characterize and quantify diffusion volume. Current methods for segmentation and quantification of diffusion volumes are largely manual and time intensive. In this paper, we explored and validated a method of automatic segmentation of diffusion volumes. This algorithm was validated against known values from previous studies. We then studied the number of angles required to accurately predict diffusion volumes. We determined accurate volumes can be determined with as few as 3 directional vectors while accurate infarct mapping requires only 7 allowing for reduced scan time.
Introduction
Stroke is the leading cause of disability and the third leading cause of death in the United States. A critical predictor of stroke severity and potential efficacy of reperfusion therapy is the degree and duration over which cerebral blood flow (CBF) is compromised. The goal of reperfusion therapy is restoration of normal CBF in hypoperfused yet viable brain tissue (ischemic penumbra). Given the complexity of quantifying collateral arterial blood supply, and its effect on true tissue perfusion [1], the volume of diffusion positive tissue, along with neurofunctional assessment score as the NIH stroke scale, are the most pragmatic, and consequently widely used tools for stroke triage. In an age of capped reimbursement and rising rate of stroke among the aging population of the world, there is an unmet need for simple, accurate and robust algorithms for triage of stroke patients. In this paper, we explore the quantification of diffusion volume in stroke. We introduce directional heterogeneity, an imaging marker specific to edema and optimize a scan protocol and segmentation algorithm for automatic quantification of infarct volume.Methods
Diffusion weighted images were acquired in canine model of acute stroke. Controlled ischemia was induced through permanent occlusion of the middle cerebral artery. Briefly, following induction, animals were anesthetized (1.5-2.0% isoflurane) and ventilated. Cardiac rhythm, end-tidal CO2, glucose, body temperature, hematocrit and arterial pressure were maintained within physiologic range. The MCA was accessed from the posterior circulation via the circle of Willis using a microcatheter (Echelon 10, EV3, Plymouth, MN) and occluded using embolic coils (Axium, EV3, Plymouth, MN) [7]. DSA images were acquired (OEC9800,GE OEC Medical Systems, Inc, Salt Lake City, UT) to confirm occlusion. Once MCA occlusion was confirmed the animal were placed in a human 3.0T MRI scanner (Philips, Achieva, Best, Netherlands) equipped with a 32 channel head coil. An MRI scan protocol consisting of anatomic, angiographic, susceptibility, perfusion, diffusion, cerebral oxygen extraction fraction and resting state fMRI were acquired. We focus here on the quantification of diffusion volumes and validation thereof. Infarct Segmentation: Cytotoxic edema (i.e. fresh infarct) is characterized by intracellular sequestration of water. We hypothesize that cytotoxic edema can be identified by loss of diffusion heterogeneity normally associated with axonal water diffusion. Therefore the angular dependence of signal in a diffusion weighted scan, if properly optimized can be used to easily, and automatically, identify cytotoxic edema in stroke. We compare three metrics of heterogeneity: Image entropy, angular heterogeneity = ||σ/μ|| and simple ADC to determine which of these metrics carries the greatest discriminating power between cytotoxic edema and viable brain. Angular Dependence: The metrics proposed carry a well-known angular dependence as the span the white matter track direction. To determine the impact on diffusion volume calculation we collected data on a series of 4 experiments where 32 uniformly distributed diffusion directions (i.e angles) were collected (FOV/Matrix = 150 mm/128, Nslices/Thickness= 20/5.0mm, b = 0, 333, 667, 1000 sec/mm2, TR/TE= 2131 ms / 71 ms, Sense factor = 2). We performed a Monte Carlo study of the angular data sets where we randomly selected sets of diffusion angles ranging from 3 to 31 angles. Sorensen-Dice Coefficients (DCE) were used to quantify the spatial accuracy as a separate metric of success. The number of angles and directions were studied.Results
We found that the variability of DWI as a function of diffusion direction provided superior discriminating power relative to entropy of the signal. (Figure 1). A threshold value for u/s of 7 was found to optimize the accuracy and a study of 6 times points throughout the growth of an infarct in 5 experiments showed that automatic segmentation agrees well with reference standard values (Auto DWI) = 1.07*( Manual DWI)+1137 mm3, r2 = 0.93). The Monte Carlo study showed that accurate volumes are calculable for as few as three diffusion directions, with errors under 5%. However, DCE coefficients indicate that no fewer than 7 directions are required to accurately map the infarct (Figure 2).Discussion/Conclusion
We have found that in addition to increased signal on DWI MRI scans, cytotoxic edema exhibits a pronounced loss of directionality. The loss of directionality serves as an additional property that allows for improved segmentation of cytotoxic edema from viable parenchyma.Acknowledgements
No acknowledgement found.References
[1] Christoforidis et al AJNR 2016.Figures
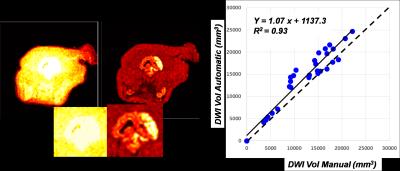
Figure 1: A
comparison between angular entropy (left) and μ/σ for
identifying diffusion positive regions in a middle cerebral artery occlusion
stroke. The μ/σ outperformed entropy and correlates well (solid
line) with manually drawn DWI volumes (right, dotted line is unity).
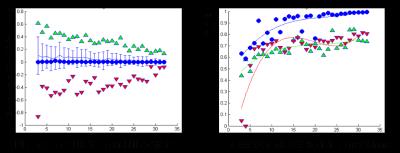
Figure 2: We
calculated the error on the automatic determination of the infarct volume in
subsets of diffusion directions (i.e. angles) ranging from 3 directions to 31
directions, compared to reference set of 32 uniformly distributed
directions. (A) Best (blue dots) and
worse (Green overestimate, Red underestimate) of volume were selected. Note
that even with as few as 3 angles infarct volumes can be accurately determined.
(B) Dice CoEfficient (DCE) shows that a minimum of 7 angles are required to
determine volume and anatomic distribution of infarct.