4578
Ischemic brain lesions and cognition in patients with aneurysmal subarachnoid hemorrhage.1Radiology, University Medical Center Utrecht, Utrecht, Netherlands, 2Rehabilitation, University Medical Center Utrecht, 3University Medical Center Utrecht, Netherlands, 4University Medical Center Utrecht, 5Radiology, University Medical Center Utrecht, Washington, DC, United States
Synopsis
A significant amount of patients with aneurysmal subarachnoid hemorrhage suffer from cognitive impairment. The exact origin of this cognitive impairment is unclear. Lesions developing in relation to the event have been hypothesized as the mechanism of action. However, literature on this topic is inconclusive. We aimed to scrutinize this by analyzing our cohort of patients. Lesions were found in 60% of patients, no relation was found with cognitive outcome, This could be due to selection bias as most of the included patients had a good or mild impaired clinical status at admission and demonstrated ‘no cognitive impairment’ at follow-up.
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) has a bad prognosis with a mortality rate of 50% and 40% of survivors suffering from a physical disability 1. In addition, a significant amount of survivors experience cognitive impairment 2. The exact origin of this cognitive impairment has not been fully established yet. Ischemic lesions developing from ictus on have been hypothesized to be the source. However, literature on this issue is scarce and inconclusive 3-7. Thereby the purpose of this study was to elucidate this topic by evaluating the relation between lesion load (number and volume of lesions) and cognitive performance.Methods
This study was approved by the institutional review board of our institution. Inclusion criteria for this study were as follows; (1) confirmed aSAH, (2) endovascular treatment of the aneurysm, (3) no aSAH in medical history, and (4) MRI performed in the subacute phase (3-21 days after ictus). Imaging was performed on either a 1.5 or 3T system (Philips, Best, The Netherlands) with a body coil as transmitter and an 8-channel head coil as signal receiver. The imaging protocol consisted of a T2 fluid-attenuated inversion recovery (T2- FLAIR) and a diffusion-weighted imaging (DWI) sequence. Scan parameters of the T2-FLAIR and DWI sequence were TR-TI-TE= 10000-2800-140/120 ms and TR-TE=3348/3015-98/68 ms with 2 b-factors (0 and 1000 s/mm2), respectively. Lesion load and location was scored blinded by an experienced neuroradiologist through a simultaneous assessment of the T2-FLAIR and DW images. Lesion volume was assessed by manually outlining the lesions on either the T2-FLAIR or DW images using Picture Archiving and Communication System (PACS) software (Sectra AB, Linköping, Sweden). Neuropsychological assessment covered the 6 cognitive domains; language, memory, executive function, attention, processing speed and visuospatial function. Patients were included in the neurocognitive outcome analysis when (1) assessment was performed >21 days after ictus, (2) a minimum of 5 out of the 6 domains were evaluated with more than 60% of all tests per domain completed. Results of the assessment were transformed into z-scores which denote the deviation from a control group (age: 58±16 years, 63% females), this transformation was done per test, per domain, and an overall z-score was created. The following categories were drawn: (1) ‘no impairment’: z-score = >-1 sd, (2) ‘mild impairment’: z-score = (-1) –(-2) sd, (3) ‘severe impairment’: z-score = <-2 sd. Statistical analysis was performed with SPSS 21.0 (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY:IBM Corp.).Results
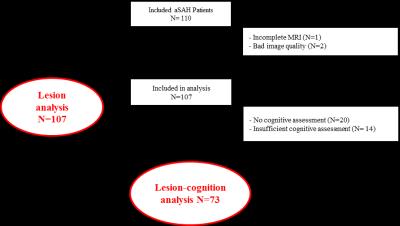
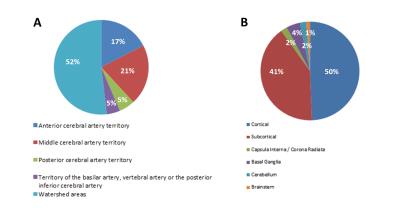
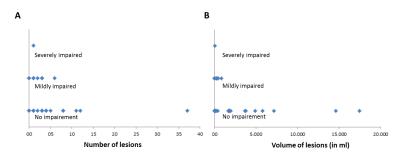
A flowhart of the data-analysis enrollment process is given in Figure 1. Baseline characteristics of the subjects included in the data-analysis are given in Table 1, 90% of patients had either a good or mildly impaired clinical status at admission as evaluated by the PAASH score (a derivative of the Glasgow Coma Scale). Lesions were detected in 61% of the patients with a median (range) number of lesions of 3 (1-37) per patient. The mean (±sd) volume of lesions per patient was 2.4 (±6.1) ml. The median volume per lesion was 0.2 (0.0009-34.8). The lesions were mainly found in the watershed areas (52%, Figure 2A) and (sub)cortical (91%, Figure 2B). Neuropsychological assessment found ‘no impairment’ in 84% of the patients. No significant relation was found between presence of lesions, number of lesions, lesion volume and cognitive outcome in any of the 6 neurocognitive domains or in overall cognitive outcome (p >0.1).Discussion and conclusion
In this study we found no correlation between lesion load and cognitive outcome in a cohort of aSAH patients of whom most (84%) did not demonstrate cognitive impairment at follow-up when assessed with a neuropsychological test battery. Most of the patients included in this study presented with either good or mild impaired clinical status (90%) at admission which could explain their mild clinical course. The number of lesions did correspond with earlier published data, however, the lesion volume was lower 4. In conclusion, our data does not elucidate cognitive impairment after aSAH due to a selection bias. This selection bias is thought to be caused by relative contraindications for MRI in clinically unstable aSAH patients who are most likely also the patients who will experience most cognitive problems at follow-up.Acknowledgements
No acknowledgement found.References
1. Zacharia BE, Hickman ZL, Grobelny BT, et al. Epidemiology of aneurysmal subarachnoid hemorrhage. Neurosurg Clin NAm. 2010;21:221-233
2. Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41:519-536
3. De Marchis GM, Pugin D, Meyers E, et al. Seizure burden in subarachnoid hemorrhage associated with functional and cognitive outcome. Neurology. 2016;86:253-260
4. Frontera JA, Ahmed W, Zach V, et al. Acute ischaemia after subarachnoid haemorrhage, relationship with early brain injury and impact on outcome: A prospective quantitative mri study. J Neurol, Neurosurg Psychiatry. 2015;86:71-78
5. Sato K, Shimizu H, Fujimura M, et al. Acute-stage diffusion-weighted magnetic resonance imaging for predicting outcome of poor-grade aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2010;30:1110-1120
6. Shimoda M, Hoshikawa K, Shiramizu H, et al. Clinical implications of subarachnoid clots detected by diffusion-weighted imaging in the acute stage of aneurysm rupture. Neurol Med Chir. 2010;50:192-199 7. Shimoda M, Hoshikawa K, Shiramizu H, et al. Early infarction detected by diffusion-weighted imaging in patients with subarachnoid hemorrhage. Acta neurochir. 2010;152:1197-1205
Figures