4565
Associations between white matter lesions, age, and 4D flow MRI hemodynamics in 69 patients with Sickle Cell Disease1Radiology, Academic Medical Center, Amsterdam, Netherlands, 2Pediatric Hematology, Emma Children's Hospital, Academic Medical Center, Amsterdam, Netherlands, 3Sunnybrook Research Institute, Toronto, Canada, 4Cardiology, Children's Hospital Los Angeles, Los Angeles, CA, United States, 5Biomedical Engineering and Physics, Academic Medical Center, Amsterdam, Netherlands, 6Internal Medicine, Hematology, Academic Medical Center, Amsterdam, Netherlands
Synopsis
Hemodynamic parameters such as wall shear stress(WSS) may be adversely affected in Sickle Cell Disease(SCD). Vaso-occlusion is a common complication leading to ischemic organ damage. We investigated how impaired hemodynamics (velocity, WSS, flow and lumen area) relate to ischemic white matter lesions(WMLs). Our aim was to quantify age-related changes in hemodynamics and to investigate their relationship with WMLs. 14 controls and 69 patients underwent 4D-flow MRI. We assessed intracranial velocity, WSS, flow and lumen area in the circle of Willis. We show that 4D-flow parameters are decreased in patients with WMLs, but age is an important factor in this relationship.
Introduction
Wall shear stress (WSS) is the force exerted on vascular endothelial cells by blood flow, and is an important factor in autoregulation. In Sickle Cell Disease (SCD), adhesive forces between blood cells and their aggregates with the vessel wall facilitate vaso-occlusion1 and require high shear stress for removal from the endothelial wall2. In parallel to painful vaso-occlusive episodes, at least 30% of SCD patients have ischemic cerebral white matter lesions (WMLs)3. We investigated the relationship between impaired hemodynamics (low velocity, WSS, flow) and WMLs. Hemodynamic parameters are known to be age-dependent4, hence our aim was also to quantify the age-related range of intracranial hemodynamic parameters velocity, flow, WSS, and lumen area, in patients and controls. We hypothesized that patients with WMLs have impaired hemodynamics on the aforementioned parameters measured with 4D-flow MRI.Methods
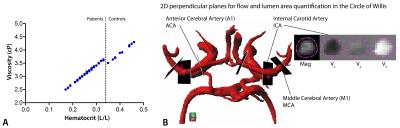
14 healthy controls (median[IQR] age: 20[18–43], 8 male)and 69 patients (median[IQR] age: 15[11–23], 42 male) were included based on genotype(HbSS/HbSb0), informed consent, and age(8-60years). Hematocrit(Hct) measurements from blood were used to calculate patient-specific viscosity values(figure 1A), based on shear rates5 derived from 4D-flow MRI, and hence, 3D WSS estimation6 incorporated subject-specific blood viscosity. All subjects underwent 4D-flow MRI and patients separately also had standard clinical FLAIR MRI at 3T (Intera/Ingenia, Philips Healthcare, Best, The Netherlands) with an 8/16/32-channel receive head-coil and body-coil transmission. FLAIR MRI images were used to score white matter lesions7. 4D-flow images were acquired in the circle of Willis with retrospective cardiac gating over four cardiac phases, TE/TR=3.2/6.5ms, flip angle=15-20º, VENC=100cm/s in three principal directions, spatial resolution=0.5mm3, slices=30-36 and scan duration=5-8mins. Background phase offset correction was performed automatically on the MR system. Data post-processing included vessel segmentation in Mimics(Materialise, Leuven, Belgium) using magnitude images at the timeframe with the highest signal intensity. Data were unwrapped and time-averaged velocity, WSS and diameter values were calculated in Matlab. Segments of interest included the middle cerebral artery, anterior cerebral artery, and the proximal internal carotid artery(figure 1B). Flow and lumen area were computed in single cross-sections placed perpendicular to the vessels of interest in GTFlow software(Gyrotools, Zurich, Switzerland). Statistics included t-test and ANOVA with age as covariate for group comparisons, exponential fit to assess age-related changes associated with 4D-flow MRI parameters: velocity, WSS, flow and lumen area. Linear regression was used to model WMLs with age and WSS data, while correlation coefficients were calculated to assess the strength and direction of the relationships between age/WSS/velocity and WMLs.Results
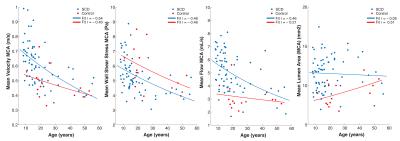
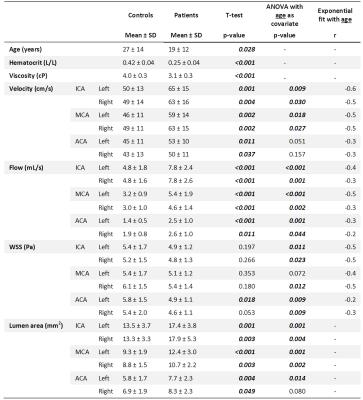
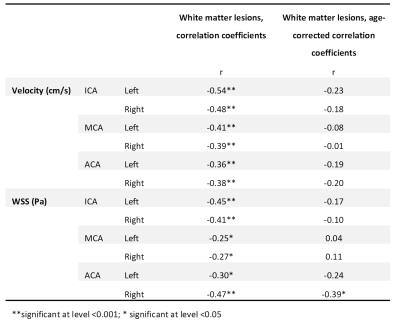
Subject characteristics are provided in table 1, which furthermore shows that time-averaged velocity, flow and lumen area were significantly higher in patients. WSS was lower in patients, but only when age was included in the ANOVA analysis. R-values for the exponential fit of 4D-flow parameters with age are given in the final column. This age-related change in 4D-flow parameters is shown in figure 2 for the MCAs. FLAIR images in figure 3A show a young patient without WMLs and an elderly patient with many WMLs. Their respective WSS maps are shown in 3B, revealing lower WSS in the older patient with more WMLs. There was a significant inverse relationship between mean WSS of the ICA, MCA and ACA vessels and WMLs, as shown in figure 3C(r=-0.43,p<0.001). WMLs were also inversely related to mean velocity of the ICA, MCA and ACA(r=-0.50, p<0.001, figure 3D). However, WMLs also correlated significantly with age (r=0.74,p<0.001,3E). ANCOVA revealed that although there were significant effects of mean velocity (p<0.001) and mean WSS (p=0.02) alone on WMLs, mean WSS was no longer significantly associated with WMLs when age was accounted for (p=0.49), similarly, mean velocity was not associated with WMLs when corrected for age (p=0.17). This is revealed in table 2, which provides correlation coefficients and age-corrected coefficients for individual vessels of interest.Discussion
Our findings show that lumen area was not associated with age in patients. Velocity, flow and WSS showed an age-dependent decline, with an initial steep decline in late childhood, as previously reported in healthy subjects4. The large variation around the age of 10 may reflect the age of high risk for overt stroke, which is reported in literature at age 9, and is associated with high Transcranial Doppler velocities8. WSS and velocity were individually associated with WMLs, but this relationship was lost when age was taken into account.Conclusion
Age is an important factor when comparing patients with controls on hemodynamic 4D-flow MRI parameters, and while white matter damage appears to coincide with low velocity and low WSS in SCD patients, age is a significant factor precluding the direct establishment of a causal relationship.Acknowledgements
Fonds Nuts Ohra (Dutch Foundation)References
[1] Switzer JA, Hess DC, Nichols FT, Adams RJ (2006) Pathophysiology and treatment of stroke in sickle-cell disease: present and future. The Lancet Neurology, 5:501-512.
[2] Barabino GA, McIntire LV, Eskin SG et al. (1987) Endothelial cell interactions with sickle cell, sickle trait, mechanically injured, and normal erythrocytes under controlled flow. Blood, 70:152-157
[3] Vermeer SE, Longstreth WT, Koudstaal PJ (2007) Silent brain infarcts: a systematic review. The Lancet Neurology, 6:611-619.
[4] Wu C, Honarmand AR, Schnell S, Kuhn R, Schoeneman SE, Ansari SA, Carr J, Markl M, Shaibaniet A (2016) Age-Related Changes of Normal Cerebral and Cardiac Blood Flow in Children and Adults Aged 7 Months to 61 Years. Journal of the American Heart Association 5(1):e002657.
[5] Detterich J, Alexy T, Rabai M, Wenby R, Dongelyan A, Coates T, Wood J, Meiselman H (2013) Low-shear red blood cell oxygen transport effectiveness is adversely affected by transfusion and further worsened by deoxygenation in sickle cell disease patients on chronic transfusion therapy. Transfusion 53: 297-305.
[6] Potters WV, van Ooij P, Marquering H, vanBavel E, Nederveen AJ. (2015) Volumetric arterial wall shear stress calculation based on cine phase contrast MRI. Journal of Magnetic Resonance Imaging 41: 505-516.
[7] Scheltens PH, Barkhof F, Leys D, Pruvo JP, Nauta JJP, Vermersch P, Steinling M, Valk J (1993) A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. Journal of the Neurological Sciences 114:7-12.
[8] Adams RJ, McKie VC, Carl EM, Nichols FT, Perry R, Brock K, McKie K, Figueroa R, Litaker M, Weiner S, Brambilla D (1997) Long-term stroke risk in children with sickle cell disease screened with transcranial Doppler. Annals of Neurology 42:699-704.
Figures