4564
Longitudinal metabolic evolution of rat cortex upon global ischemia: an ultra-short echo time 1H MRS study1Biomedical Imaging Research Center, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 2University of Geneva, Geneva, Switzerland
Synopsis
Stroke is a leading cause for disability. Metabolic evolution of transient ischemia attack might shed insights for diagnosis and prognosis. We aimed to 1H MRS study metabolic evolution of cortex before, during and immediately after 15-min complete global ischemia. Combination of MR angiography (MRA) and 1H MRS allows execution of vascular occlusion models directly in the magnet as well as proper identification and characterization of complete stroke.
Introduction
Stroke is the leading cause of disability and the third cause of death in the world. Since changes in brain metabolism are a key element in the outcome of stroke, a better understanding of brain metabolism during and after stroke might lead to novel therapeutic targets. The ability of 1H MRS to identify stroke damage, predict stroke outcome (neuronal loss or recovery) and estimate ischemic onset time according to metabolite concentration, has been previously demonstrated in studies applying a well-defined transient focal middle cerebral artery occlusion (MCAO) model in mice (1,2). These studies showed blood perfusion threshold for stroke lower than 20% and a reperfusion level higher than 50% (1,2). However, metabolite concentration changes in the brain resulting from 1) different degrees of stroke severity (depending on efficiency of blood flow occlusion and duration of MCAO), 2) restored blood flow level during reperfusion or 3) therapeutic treatments aimed to prevent stroke outcomes, are underexplored. Indeed, transient MCAO requires a filament to be inserted and removed from the MCA, which makes 1H MRS studies in rodents very challenging during and immediately after a brief period of stroke (mouse should be placed and removed from the magnet). Recently, transient MCAO has been performed in the magnet on rats using a sophisticated setup (3), but in diffusion and perfusion MRI studies only. Additionally, MCAO allows occlusion of blood flow in MCA irrigated regions only, e.g. the striatum (1, 4). Studying effects of acute stroke on other brain regions, like cortex and hippocampus, would be problematic using the MCAO model. Alternatively, it was shown that a 4-vessel occlusion model (4-VO), where both vertebral and common carotid arteries can be occluded remotely (5), results in bilateral hemispheric ischemia in rats. Application of 4-VO model has been underexplored until now, e.g. for studying stroke outcome in view of therapeutic strategies. In this study, we evaluated if localized 1H MRS at ultra-short time in combination with MR angiography (vasculature images, 6) could be applied for diagnostic purposes on rat upon global ischemia (4-VO) in a 14.1T magnet. Secondly, we assessed the longitudinal metabolic evolution of the cortex upon a short period of transient global ischemia.Methods
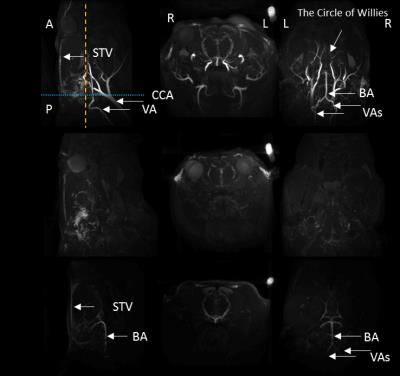
All studies were approved by the local animal care and use committee. We applied one-stage anterior approach (7) to achieve 4VO in adult male Wistar rats (300-350g). In brief, rats were anesthetized for surgical preparation under 2.5% isoflurane mixed with air and oxygen (1:1). Both vertebral arteries (VAs) were carefully occluded between cervical diapophysis C2 and C3 using a cauterizer. Common carotid arteries (CCAs) were isolated and encircled loosely with 1.5 mm-diameter inflatable vascular occluders, which were saline-filled and extended to syringes outside the magnet. Once CCAs were ligated, 4-VO could be achieved. Immediately, animal heads were carefully secured, placed in prone position and transferred in a horizontal 14.1T magnet. A quadrature coil (two 16-mm-inner-diameter loops) was placed on the top of cortex and used for MR angiography and localized 1H MRS. Once the field inhomogeneity was adjusted, 3D GRE images were acquired to cover the entire head and part of the neck for MR angiography (maximum intensity projection (MIP, 6) ). Localized 1H MR spectra were acquired using SPECIAL (1,2). Bilateral cortical tissue (1×8×4.2mm3) was defined based on anatomical images (FSE/GRE). After field homogeneity adjustment, 1H MR spectra with sufficient signal-to-noise (e.g. >8) were quantified using LCMODEL referencing the endogenous water signal from the identical voxel (80% water contents in cortex).Results and Discussion
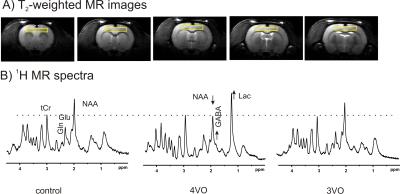
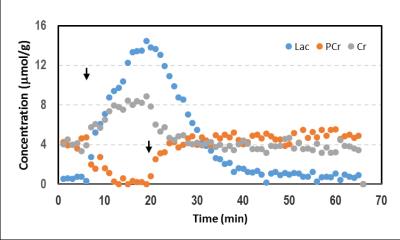
The MIP images illustrated the typical vascular structure of rat head, e.g. CCAs, VAs, BA and the circle of Willies (Figure 1). After 4-VO, the MIP images showed that nearly all signals originated from major arteries were significantly decreased. Rats showing remaining signal intensities underwent incomplete 4-VO and thus were excluded from the metabolic study, as shown in Figure 2. MRA techniques illustrated that vascular signal intensities globally decreased significantly during both 4-VO and 3-VO, but complete reduction was obtained in 4-VO only. Thus, incomplete occlusion by 3-VO is sufficient in maintaining normal cortical metabolite profile and possibly results in attenuated ischemic attack in the cortex. 1H MRS data suggested that metabolic modifications in cortical tissue occured immediately only in case of a complete global ischemia (Figure 2). Then, we studied brain metabolism before, during and after 15min 4-VO. After VAs occlusion, 1-min temporal resolution of metabolite evolution was obtained in the cortex by 1H MRS (Figure 3). Once 4-VO was achieved remotely, phosphocreatine (PCr) decreased while creatine (Cr) and lactate (Lac) increased. When reperfusion was restored by releasing the occluders, PCr recovered and Cr decreased, while lactate decreased gradually (Figure 3). These observations were consistent with depletion and recovery of oxygenation.
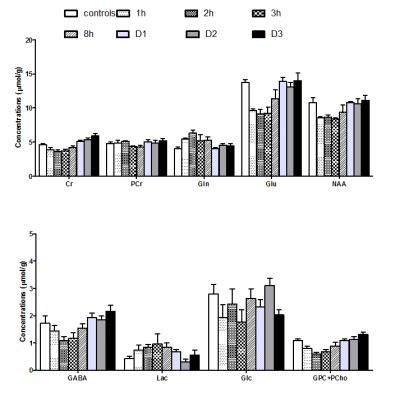
Evolution
of metabolites concentration in the brain was studied at different time points
from 1h to 3 days after 15 min 4-VO ischemia (1h, 2h, 3h, 8h, D1, D2 and D3; Figure
4). A transient elevation of glutamine (Gln) was observed after restoration of
perfusion (Figure 4) consistently with excitoxicity reported in mice after
transient focal ischemia (1).
Conclusion
4-VO model of complete brain ischemia can be applied easily and successfully in MR studies. Cortical metabolic changes can be assessed by 1H MRS during and immediately after cerebral ischemia. MRA combined with1H MRS characterization of cortical metabolic profile can be successfully used to diagnose complete global ischemia.Acknowledgements
This work was supported by the Centre d'Imagerie BioMédicale of the UNIL, UNIGE, HUG, CHUV, and EPFL and the Leenaards and Jeantet Foundations.References
1) Berthet C et al. 2011 Stroke
2) Berthet C et al. JCBFM 2014 ; 34:1848-1855
3) Li FH et al. 1998 Stroke 29:1715-1720
4) Longa EZ et al. 1989 Stroke
5) Behar KL et al. 1989 JCBFM 9:655-665
6) Beckmann N et al. JMR 1999; 140:442-450;
7) Yamaguchi M et al. Stroke. 2005; 36:2212-2214
Figures