4563
The brain metabolite change of magnetic resonance spectroscopy for evaluating he Rehabilitation therapy effect of intra-cerebral hemorrhage rat animal model1Gimcheon University, Gimcheon, Korea, Republic of, 2Gimcheon University, Gimcheon, Korea, Korea, Republic of
Synopsis
The objective of this study were to examine the brain metabolite concentration quantification change by in-vivo 1H-MRS analysis in animal hemorrhage model, and we determined the bio-marker that was shown the effect of exercise treatment in hemorrhage disease. No significant difference in the concentration levels of experimental and control group were observed (p=0.839). There was great significance in revealing that (Glu+Gln)/tCr value was increased, and tCho/tCr concentration level was decrease applying exercise treatment methods on hemorrhage animal model. Therefore, the metabolite concentration change of (Glu+Gln)/tCr and tCho/tCr can be used as a powerful bio-marker that represented an exercise treatment in hemorrhage patients.
Purpose
The objective of this study were to examine the brain metabolite concentration quantification change by in-vivo 1H-MRS analysis in animal hemorrhage model, and we determined the bio-marker that was shown the effect of exercise treatment in hemorrhage disease.Methods
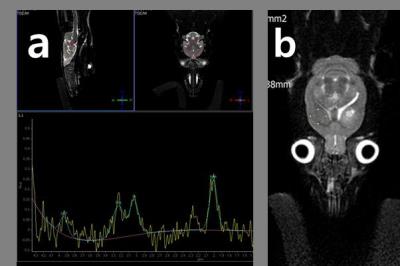
The animal experiments were in accordance with the National Institutes of Health Guidelines for Animal Research “Guide for the Care and Use of Laboratory Animals” and were conducted according to protocol. The 6-weeks-old 24 SpragueDawley rats weighing 100150g were housed with ad libitum access to water with weight matched. The rats were anaesthetized with chloral hydrate (400mg/kg, i.p.) and placed in a stereotactic frame. A needle (30G, Carpule®, Heraeus Kulzer, Germany) was implanted into the left striatum (3.5mmlateral and 7mm deep from the bregma), according to the atlas of Paxinos and Watson1. Five minutes after the insertion of the needle, collagenase (type IV-S, Sigma C1889, 0.12U in 1μ NaCl 0.9%) was injected over the next 5min using a pump (Precidor®, Infors HT, Switzerland). The needle was left in place for another 5min and then slowly removed and the wound was sutured. The experimental group 12rats were conducted treadmill (JD-A-09 type, jEUNGDO Bio & Plant Co., Ltd.,Korea) training. The training program of this research was implemented in 2 weeks with 55 to 85% of V02max and during determined period 15min one a day. The control group animals were housed under standard conditions (2/3 rats per cage) with a 12 hr light/dark cycle and allowed access to food and did not give the any medical behavior. All MRI and 1H-MRS experiments were performed on a 3.0Tesla MRI scanner (Achiva Tx 3.0 T; Philips Medical Systems, Netherlands) with a maximum gradient of 200 mT/m using a 4-channel animal coil (CG-MUC18-H300-AP, Shanghai Chenguang Medical Technologies Co., Ltd., China). During brain MRS and imaging, all hemorrhage model rats were anesthetized with isoflurane/air at 1.0 to 1.5% via a nose cone with respiratory monitoring. Using T2-weighted fast spin echo, whole brain images were acquired in three transverse axial (FOV 60 mm×60 mm, slice thickness = 1 mm), coronal (FOV 60 mm×60 mm, slice thickness = 1 mm), and sagittal (FOV 60 mm×60 mm slice thickness = 1 mm) planes to localize voxels or volume of interest for MRS. We use a point-resolved spectroscopy (PRESS) sequence for localized 1H-MRS with TR = 1,500 msec, TE = 35 msec, NEX = 64 and total scan time = 10 minute. A 8×6×6 mm3 voxel was placed within a whole brain parenchyma with hemorrhage area. We applied iterative VOI shim. The water signal of each VOI was suppressed by variable pulse power and optimized relaxation delays before the scan2. The signal was shimmed to a line width of lipid (4 to 8 Hz) over VOI. The 1H-MRS raw data were analyzed using LCModel software (version 6.31H, Stephen W. Provencher). The 1H-MRS raw data were analyzed using LCModel software (version 6.31H, Stephen W. Provencher). The integrating areas under peaks were measured as follows: glutamate (Glu), glutamine (Gln), Choline-containing Compounds (tCho, phosphoryl choline + glycerophosphochline), N-Acetyl Aspartate (NAA), N-Acetyl Aspartyl Glutamate (NAAG). Less than 15% standard deviation (%SD) of metabolite quantification data was allowed. The %SD called the Cramér-Rao lower bound of useful reliability indicators was used for error estimates. All of MRS brain metabolite data were calculated total creatine (creatine+phosphocreatine, tCr) ratio (metabolite concentration/tCr concentration), because the tCr use the basic metabolite material in MRS analysis. We performed normal distribution test (Kolmogorov-Simrnov test) firstly, and we was conducted independent sample t test with a significance level of p<0.05. All of the results were expressed as mean ± standard deviation.Results
The concentration value of (Glu+Gln)/tCr at experimental group after applying the exercise treatment was 1.838±0.606, which was significantly (p=0.021) higher than control group (1.270±0.283). The tCho/tCr concentration value of experimental group was 0.267±0.012, which was significantly (p=0.000) lower than control group (0.352±0.051). The NAA/tCr and NAAG/tCr of concentration could not separate, the (NAA+NAAG)/tCr concentration value for experimental and control group were 1.258±0.226 and 1.2742±0.093 respectively.Discussion
The Glu concentration of experimental group (1.340±0.197) was higher than control group (1.153±0.132), but there was statically insignificant (p=0.067). No significant difference in the concentration levels of experimental and control group were observed (p=0.839). There was great significance in revealing that (Glu+Gln)/tCr value was increased, and tCho/tCr concentration level was decrease applying exercise treatment methods on hemorrhage animal model.Results
The metabolite concentration change of (Glu+Gln)/tCr and tCho/tCr can be used as bio-marker that represented an exercise treatment in hemorrhage patients.Acknowledgements
This work was wupported by the 2016 Gimcheon University Research Grant (gc16005).References
1. Thompson IM, Watson RA, Rodriguez FR: TURP followed by intraoperative hemorrhage incontinence. Mil Med 1987, 152(1):50-51.
2. Tkac I, Starcuk Z, Choi IY, Gruetter R: In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med 1999, 41(4):649-656.
Figures