4562
Evaluation of hemodynamic impairments in healthy elderly participants and patients with high-grade unilateral carotid artery stenosis1Department of Neuroradiology, Technical University of Munich, Munich, Germany, 2PET center, Institute of Radiopharmaceutical Cancer Research, Helmholtz-Zentrum Dresden-Rossendorf, Dresden, Germany, 3Philips Healthcare, Best, Netherlands, 4Philips Research, Hamburg, Germany, 5Philips Healthcare, Hamburg, Germany, 6Department of Radiation Oncology and Radiotherapy, Charité Berlin, Berlin, Germany, 7Department of Radiology, Technical University of Munich, Munich, Germany, 8TUM Neuroimaging Center, Technical University of Munich, Munich, Germany, 9Clinic for Neurology, Technical University of Munich, Munich, Germany
Synopsis
Internal carotid-artery stenosis (ICAS) causes complex and not yet well understood physiological impairments. We present preliminary data from an ongoing clinical study in ICAS patients and healthy, age-matched participants. The major aims were to evaluate the reliability of a multimodal MRI-protocol and investigate physiological changes. For ICAS patients, regionally impaired vascular-reactivity as well as hypo-perfusion were found. In accordance with literature, we did not find ICAS-induced changes in oxygen extraction on group level. The presented preliminary results thus imply successful application of multimodal MRI methods and are highly promising with respect to gaining a deeper insight into ICAS-related physiological changes.
Purpose
Severe internal carotid-artery stenosis (ICAS) is a major public health issue, as it is known to cause cognitive impairments1,2 and accounts for approximately 20% of all strokes3. Hemodynamic changes by cerebrovascular diseases have been intensively investigated4-8. However, the relations among hemodynamic impairments and compensation mechanisms are still not well understood, especially in subacute or chronic disease. Thus, we propose a combination of three different MR-based methods, accounting for cerebrovascular reactivity (CVR) by breathhold-fMRI, cerebral blood flow (CBF) by pseudo-continuous arterial spin labeling (pCASL) and relative oxygen extraction fraction (rOEF) (Fig.1). The latter is mapped by the multi-parametric quantitative BOLD (qBOLD) approach12,13 which has been successfully applied in patients with glioma14 and stroke15, but requires further clinical evaluation. Here, we present preliminary data from a clinical study in patients with unilateral ICAS and healthy age-matched participants. The major aims are to evaluate the reliability of the proposed methods and gain deeper knowledge of involved physiological changes.Methods
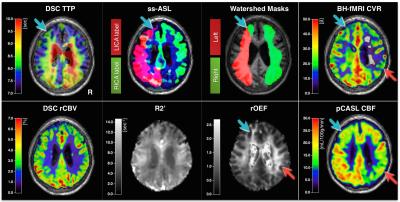
In the ongoing clinical study, 52 subjects (29 healthy: 70.3±4.7y, 13 males; 23 patients: 70.5±6.8y, 15 males, with asymptomatic unilateral high-grade ICAS, NASCET>70%) underwent MRI on a clinical Philips 3T Ingenia MR-Scanner (Philips Healthcare, Best, Netherlands), using a 16ch head/neck-coil for clinical- and 32ch head-coil for functional-imaging. The protocol comprised measurements of CVR, CBF and rOEF (Fig.1). CVR was measured by a breathhold-fMRI scheme16 using single-shot EPI-readout (voxelsize 3x3x3mm3, 38 slices, Multiband/SENSE=2/2, TE=30ms, TR=1200ms, acq.time=5:48min). Processing was implemented using a data-driven approach9. Non-invasive perfusion mapping was performed by pCASL using a segmented 3D GraSE-readout (voxelsize 2.7x2.8x6mm3, 16 slices, TE=7.4ms, TR=4403ms, label duration=1800ms, PLD=2000ms, background suppression, 3 repetitions, including proton-density-weighted M0 normalization, acq.time 5:43min). CBF was calculated following the ISMRM perfusion study group white-paper with λ=0.9ml/g and labeling efficiency α=0.8510. rOEF-mapping (voxelsize 2x2x3mm3, 30 slices) was performed using the multi-parametric qBOLD approach12,13 acquiring a multi-echo GraSE (8 echos, TE1=∆TE=16ms, TR=8971ms, acq.time 2:24min) and multi-GE sequence (12 echoes, TE1=∆TE=5ms, TR=1950ms, α=30°, rapid flyback gradients, acq.time 6:08min) for T2- and T2*-mapping12. rOEF-calculation requires measurement of relative cerebral blood volume (rCBV), obtained by dynamic susceptibility contrast (DSC)11 using a bolus-injection of 15ml Gd-DTPA and single-shot GE-EPI-readout (TE=30ms, TR=1516ms, α=60°, 80 repetitions) after a carotid artery angiography with 17ml Gd-DTPA. Processing was performed with SPM1217 and custom Matlab programs18. For each participant, individual masks of watershed areas were defined for both hemispheres using DSC-based Time-to-Peak (TTP) maps. In selected cases, superselective-pCASL (ss-ASL) of both internal carotid arteries was used to verify individual collateralizations19. Parameter mean values were compared by Bland-Altman plots20.
Results
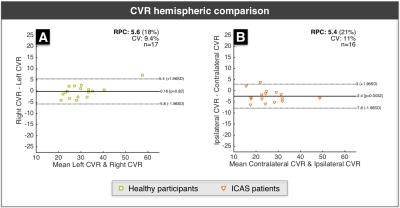
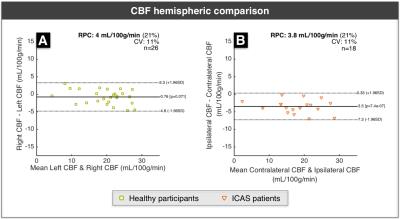
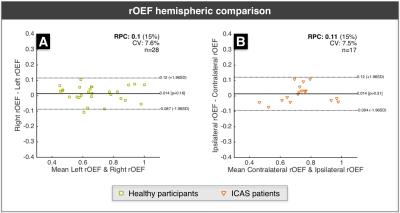
Figure 2 shows exemplary data from a right-sided ICAS patient. For statistical analysis, low quality parameter maps were excluded according to ratings (SK, CP)22. CVR revealed good symmetry in healthy participants, but showed ipsilateral decreases for ICAS patients (Fig.3). CBF-maps show a similar behavior, demonstrating a clear hypo-perfusion within the affected hemisphere of ICAS patients (Fig.4). Analysis of rOEF maps yielded good symmetry between hemispheres for both healthy participants and in ICAS patients (Fig.5). Thus, our preliminary data demonstrate ICAS induced ipsilateral CVR and CBF decreases while rOEF appear unaffected.
Discussion
In healthy participants, our results point to perfectly symmetric CVR and slight CBF variations between hemispheres. Underlying causes for these minor asymmetries may be individual vascularization patterns as well as microvascular changes within our elderly cohort23. Regarding ICAS patients, we observed ipsilateral hypo-perfusion for all patients and ipsilateral CVR impairment in 14 of 16 cases. Hypo-perfusion as well as chronic vasodilation may thus be characteristic for severe ICAS. Even though focal rOEF increases could be suspected in single patients (Fig.2), a global symmetry between hemispheres prevails (Fig.5). This contradicts expectations raised by findings of increased rOEF in acute stroke7,15,24, but agrees with recent results in stenosis patients4. Consequently, the presented qBOLD approach has to be further evaluated with increasing the sample size and additional analyses. Here, we restricted the analysis to GM to avoid confounds due to orientation effects within WM12, but a careful analysis of perfusion and rOEF in WM might be necessary to fully assess vascular impairment in ICAS patients.Conclusion
These preliminary results show that breathhold-fMRI and pCASL can detect the expected hemodynamic symmetry in healthy participants. In ICAS patients, breathhold-fMRI and pCASL enable the reliable detection of the expected impairments of CVR and perfusion. With regard to rOEF, there is no easy conclusion: while it appears unchanged on a global scale, single cases seem to show more focal changes, clearly requiring further investigations. In summary, our results show that multimodal MRI can assist in gaining a deeper knowledge on physiological changes induced by cerebrovascular diseases.
Acknowledgements
The authors acknowledge support by the Friedrich Ebert Stiftung and Leonhard-Lorenz Stiftung.References
1: Yue W, Wang A, Ju Y et al. Association between Carotid Artery Stenosis and Cognitive Impairment in Stroke Patients: A Cross-Sectional Study. PLOS ONE 11(1) (2016): e0146890.
2: Martinic-Popovic I, Lovrencic-Huzjan A, Demarin V et al. Advanced Asymptomatic Carotid Disease and Cognitive Impairment: An Understated Link? Stroke Research and Treatment 2012: 981416.
3: Petty GW, O'Fallon WM & Wiebers DO et al. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke, 30 (1999): 2513-2516.
4: Bouvier J, Detante O, Tahon F, et al. Reduced CMRO2 and cerebrovascular reserve in patients with severe intracranial arterial stenosis: A combined multiparametric qBOLD oxygenation and BOLD fMRI study. Human Brain Mapping 36(2) (2015) 695-706.
5: Vakil P, Lee JJ, Carroll TJ et al. Cerebrovascular Occlusive Disease: Quantitative Cerebral Blood Flow Using Dynamic Susceptibility Contrast MR Imaging Correlates with Quantitative H2[15O] PET. Radiology 266(3) (2013): 879-886.
6: Buxton RB. Interpreting oxygenation-based neuroimaging signals: the importance and the challenge of understanding brain oxygen metabolism. Front Neuroenergetics 2 (2010): 8.
7: Derdeyn CP, Videen TO, Powers WJ et al. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain 125 (2002): 595-607.
8: Derdeyn CP, Videen TO, Grubb RL, Powers WJ. Comparison of PET oxygen extraction fraction methods for the prediction of stroke risk. J Nucl Med, 42 (2001) 1195-1197.
9: Vondrácková L, Krukowski P, Petr J er al. Data-driven model for evaluation of cerebrovascular-reserve measurement with hypercapnia BOLD. Proc. Intl. Soc. Mag. Reson. Med. 24 (2016): 3801.
10: Alsop DC, Detre JA, Golay X et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic Resonance in Medicine 73(1) (2015): 102-116.
11: Kluge A, Lukas M, Preibisch C et al. Analysis of three leakage-correction methods for DSC-based measurement of relative cerebral blood volume with respect to heterogeneity in human gliomas. MRI 34(4) (2016): 410-421.
12: Hirsch NM, Zimmer C & Preibisch C et al. Technical considerations on the validity of blood oxygenation level-dependent-based MR assessment of vascular deoxygenation. NMR Biomed, 27 (2014): 853-862.
13: Christen T, Schmiedeskamp H, Zaharchuk G et al. Measuring brain oxygenation in humans using a multiparametric quantitative blood oxygenation level dependent MRI approach. MRM 68(3) (2012): 905-911.
14: Toth V, Forschler A, Hirsch NM, den Hollander J, Kooijman H, Gempt J, Ringel F, Schlegel J, Zimmer C & Preibisch C. MR-based hypoxia measures in human glioma. J Neurooncol, 115 (2013): 197-207.
15: Gersing AS, Ankenbrank M, Preibisch C et al. Mapping of cerebral metabolic rate of oxygen using dynamic susceptibility contrast and blood oxygen level dependent MR imaging in acute ischemic stroke. Neuroradiology 57(12) 2015): 1253-1261.
16: Pillai J, Mikulis DJ. Cerebrovascular Reactivity Mapping: An Evolving Standard for Clinical Functional Imaging. American Journal of Neuroradiology 36 (2015): 7-13.
17: Statistical Parametric Mapping software (SPM12) Version 6225 by UCL, London, UK: www.fil.ion.ucl.ac.uk/spm.
18: MATLAB and Statistics Toolbox Release 2013a, The MathWorks, Inc., Natick, Massachusetts, United States.
19: Griese V, Kaczmarz S, Preibisch C et al. Analysis of cerebrovascular watershed-areas in patients with high-grade carotid artery stenosis using DSC-based time-to-peak (TTP), ISMRM 2017 abstract submitted.
20: Bland-Altman and Correlation Plot software, Ran Klein 2010: http://www.mathworks.com/matlabcentral/fileexchange/45049-bland-altman-and-correlation-plot. Assesed 09.Nov 2015.
21: Vinci software, Max-Planck-Institut für neurologische Forschung, Cologne, Germany: http://www.nf.mpg.de/vinci3/. Accessed 09.Nov 2015.
22: Kaczmarz S, Göttler J, Preibisch C et al. Evaluation of perfusion and hypoxia parameters in healthy subjects and patients with high-grade carotid artery stenosis, Proc. Intl. Soc. Mag. Reson. Med. 24 (2016): 4383.
23: Preibisch C, Sorg C, Alexopoulos P et al. Age-related cerebral perfusion changes in the parietal and temporal lobes measured by pulsed arterial spin labeling. JMRI 34(6) (2011): 1295-1302.
24: Yamauchi H, Konishi J & Shio H et al. Significance of increased oxygen extraction fraction in five-year prognosis of major cerebral arterial occlusive diseases. J Nucl Med, 40 (1999) 1992-1998.
Figures

Figure 1: Overview of analyzed modalities and resulting parameters. Breathhold-fMRI (BH-fMRI) for CVR-mapping consists of an EPI time-series with five periods of alternating normal-breathing and 15sec breathhold to induce hypercapnia16. The applied data-driven analysis identifies predefined vascular territories showing the highest correlation between BOLD-signal (blue) and respiratory-response-function (red). Using this, a new regressor is defined and applied to the whole brain, generating correlation-based CVR-maps9. CBF-maps are acquired by pCASL using 3D-GraSE with a normalization image according to recent recommendations10. The multi-parametric qBOLD approach requires DSC-based rCBV-maps and T2- and T2*-quantification to calculate rOEF11,12.