4561
Evaluating brain tissue oxygen extraction fraction changes following transfusion therapy using TRUST MRI in adults with sickle cell anemia1Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, United States, 2Pediatrics - Division of Pediatric Neurology, Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
Brain tissue oxygen extraction fraction (OEF) and cerebral blood flow (CBF) have been identified as potential imaging biomarkers for triaging adults with sickle cell anemia (SCA) for aggressive blood transfusion therapy for stroke prevention; however, little is known regarding how tissue-level hemodynamics are affected by transfusions. We utilized noninvasive MRI methods to assess OEF and CBF before and after transfusions in adults with SCA. Our results showed that OEF significantly reduces after transfusion and that this reduction parallels increases in blood oxygen content, while CBF is unchanged.
Introduction
Sickle cell anemia (SCA) is a genetically-inherited blood disorder which results in elevated risk of arterial steno-occlusion and stroke. Chronic transfusion therapy may be prescribed in adults with SCA for secondary stroke prevention, but a strategy for stratifying these patients for aggressive therapy according to stroke risk has not been identified. Brain tissue oxygen extraction fraction (OEF) and cerebral blood flow (CBF) have been identified as potential imaging markers for triaging patients for personalized therapies1; however, little is known regarding how tissue-level OEF and CBF are affected by blood transfusions. Therefore, the purpose of this study was to utilize arterial spin labeling (ASL) and T2-relaxation-under-spin-tagging (TRUST) MRI2,3 to quantify brain tissue CBF and OEF, respectively, before and after blood transfusion in adults with SCA. The mechanistic model for how hemo-metabolic parameters are expected to be altered in progressive stages of SCA is shown in Figure 1. Since transfusions should increase oxygen carrying capacity, we hypothesize that OEF reduces following transfusion and that this reduction consequently parallels increases in blood hematocrit, while CBF changes less owing to parenchyma operating closer to maximal autoregulatory capacity regardless of transfusion.Methods
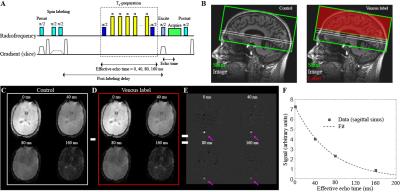
Experiment. SCA adult volunteers (n=10) provided informed consent and were scanned at 3T (Philips) at two distinct time points: 1) late in their transfusion cycle when blood hematocrit was near nadir and 2) within 7 days of receiving a blood transfusion. Participants consisted of adults (age 16 and over) with SCA genotype hemoglobin SS or Sβ0 thalassemia. pCASL data were acquired with Hanning-windowed RF pulses with post-labeling delay=1900ms (spatial resolution=3x3x7 mm3; TR/TE = 3675/13 ms; averages=20). TRUST data were acquired (TR/TE=1978/3.6 ms; spatial resolution=3.4x3.4x5 mm3) at a location 20 mm superior to confluence of the sinuses from an imaging slice containing the superior sagittal sinus. Control-label pairs were acquired at four effective echo times (eTE) of 0, 40, 80, and 160 ms with and without RF inversion of venous blood water during label and control acquisitions, respectively. Arterial oxygen saturation (Ya) was measured using peripheral pulse oximetry, and blood hematocrit was measured through a blood draw on the day of imaging.
Analysis. TRUST data were quantified2 as shown in Figure 2 by identifying the superior sagittal sinus in the pair-wise subtracted images as the four voxels with the greatest differences between control and label. A mono-exponential decay model was fit to these data across the four eTEs, and Carr-Purcell-Meiboon-Gill (CPMG) T2 was calculated. This CPMG-T2 was then converted to venous oxygen saturation (Yv) using a relationship between blood water T2, blood hematocrit, and Yv. Yv was then used, along with Ya, to compute OEF as (Ya-Yv)/Ya. CBF images were derived from the pCASL data utilizing the simplified kinetic model proposed by the ISMRM perfusion study group4 and gray matter CBF was computed for each participant. Wilcoxon signed-rank tests was applied to determine differences of study measurements at two-sided significance level of 0.05. Spearman’s correlation testing was applied to determine relationships between study measurements at a significance level of 0.05.
Results
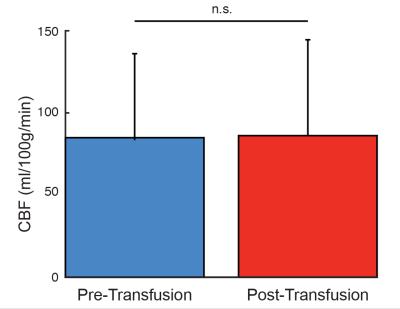
OEF reduced post-transfusion in 9/10 subjects (Figure 3B), and the means ± standard deviations of pre-transfusion and post-transfusion OEF (Figure 3A) were 0.52±0.09 and 0.42±0.06, respectively (p<0.01). Changes in post-transfusion OEF from pre-transfusion OEF were significantly correlated (ρ=-0.69; p<0.05) with changes in post- and pre-transfusion blood hematocrit (Figure 3C). In contrast, there were no significant change between the pre-transfusion and post-transfusion CBF values (Figure 4).Discussion
Brain tissue OEF and CBF have been identified as a potential imaging marker for stratifying adults with SCA to aggressive blood transfusion therapy, but the manner in which OEF and CBF are affected by blood transfusions is unknown. In this study, we have shown that OEF may reduce following transfusion and that this change parallels changes in blood hematocrit, which is increased due to transfusion. Physiologically, this implies that blood transfusions have little or no effect on CBF in many patients, as it is likely that these patients are in a chronic state of maximal autoregulatory capacity and transfusion operates only to improve oxygen carrying capacity. Future work involves investigating the lack of observed changes in CBF due to transfusions using cerebrovascular reactivity imaging.Conclusion
Brain tissue OEF significantly may reduce following blood transfusion in adults with SCA, and this reduction parallels increases in blood oxygen content, while CBF may be unaffected by transfusion in many patients.Acknowledgements
No acknowledgement found.References
1. Jordan LC, Gindville MC, Scott AO, et al. Noninvasive imaging of oxygen extraction fraction in adults with sickle cell anemia. Brain. 2016;139:738-750.
2. Lu H, Ge Y. Quantitative evaluation of oxygenation in venous vessels using t2-relaxation-under-spin-tagging mri. Magn Reson Med. 2008;60:357-363
3. Lu H, Xu F, Grgac K, et al. Calibration and validation of trust mri for the estimation of cerebral blood oxygenation. Magn Reson Med. 2012;67:42-49
4. Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion mri for clinical applications: A consensus of the ismrm perfusion study group and the european consortium for asl in dementia. Magn Reson Med. 2015;73:spcone.
Figures