4558
MR Fingerprinting in paediatric neuroradiology: our initial experience1Università di Pisa, Pisa, Italy, 2IMAGO7 Research Institute, 3IRCCS Stella Maris, Pisa Italy
Synopsis
In paediatric neuroimaging, young children have often to be sedated in order to obtain diagnostic MRI images. Magnetic Resonance Fingerprinting (MRF) is a potential alternative to sedation in children, as it achieves a fast exam with reduced sensitivity to patient motion. MRF acquisitions can be used to acquire a fully-quantitative anatomical exam in less than five minutes at a standard resolution. We performed a preliminary evaluation of MRF in 15 paediatric patients, acquiring both the standard protocol and MRF at 1.5T. Detection of brain alterations was possible, if present, in all patients. Only a few small lesions were unrevealed. MRF could be a promising tool for a fast and diagnostic exam in children, and due to its low sensitivity to motion it has the potential to allow exams without sedation.
Purpose
Magnetic Resonance Imaging (MRI) is the neuroradiological method of choice in children, thanks to the absence of harmful effects from ionizing radiation and the possibility to perform follow-up exams. However, MRI exams often have lengthy scan times and image quality is highly dependent on the absence of patient motion. To avoid uncontrolled motion and the anxiety derived from long examinations, young children are often sedated during the MRI exam. However, sedation represents an extra procedure, carrying possible adverse effects, procedural complications and additional cost. Producing diagnostic, artefact-free images in a short time in children without sedation is an unmet need.
MR fingerprinting (MRF) is a novel technique which is highly promising for providing a fast and motion-insensitive radiological assessment in children. Our aim was to perform a preliminary evaluation of MRF for detecting brain alterations in a group of paediatric patients affected by several neurological or neuropsychiatric diseases.
Methods
Fifteen patients older than two years underwent a brain MRI exam by using a 1.5T GE Signa Horizon LX 1.5T MR system equipped with a 8 channel head coil (Milwaukee, Wisoconsin, USA). For each patient, the standard protocol and a whole-brain SSFP MRF sequence [1] were acquired (acquisition parameters are shown in Figure 1). MRF Parameter maps were used to generate synthetic contrast images matching the conventional images of the standard protocol.
The standard protocol included a T1-weighted gradient-recalled echo sequence, a T2-weighted sequence and an axial fluid-attenuated inversion recovery sequence. All images were evaluated only in the axial plane. Two experienced neuro-radiologists blinded to diagnosis assessed both groups of images, MRF at first. The physicians were asked to record any brain abnormalities observed in the quantitative maps and synthetic contrasts, and then in the standard images. The ability to detect brain signal changes and lesions using MRF was assessed using conventional MRI as gold standard.
Results
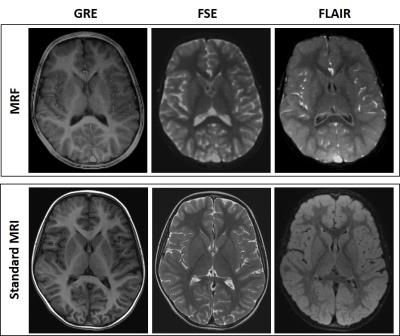
The MRF exam was performed in less than four minutes, while the standard examination took 15 minutes on average. Synthetic images from MRF had a similar appearance to conventional images, although with a slightly reduced anatomical detail, and had hyperintense vessels (see Figure 2).
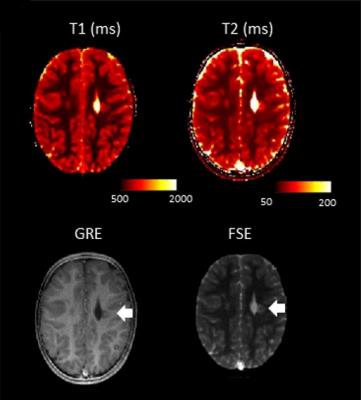
In addition to the synthetic contrast images, we also observed the quantitative maps, which also showed alterations in the same locations (see Figure 3).
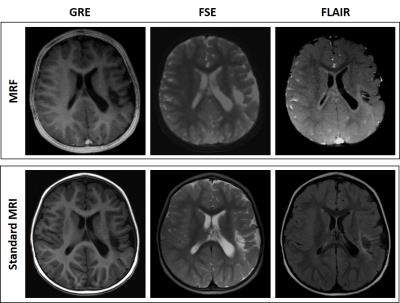
The group of patients investigated had heterogeneous medical history and final diagnosis. There were two patients with perinatal hypoxic-ischemic encephalopathy, one patient with a previous stroke (see Figure 4), one patient with microcefalia, dysmorphic lateral ventricles and cerebellar atrophy, one patient with leukoencephalopathy with cysts and calcifications, one patient with hypo myelination, one patient with demyelinating lesions, one patient with malformation of cortical development and two patients with arachnoid cysts. Five patients had no brain disease both at MRF and at standard imaging.
In all patients, the assessment of quantitative maps and synthetic contrasts allowed the detection of brain signal abnormalities and lesions, if any, compared to the corresponding images of the standard protocol. In a few cases, the evaluation of MRF images underrated the number or the extension of the signal alterations. More in detail, the detection of very small nodules of heterotopic grey matter failed, and the gliotic alterations were underestimated.
Discussion
The wide spectrum of paediatric diseases included in this study, including malformations, white matter diseases, post-ischaemic and hypoxic-ischaemic lesions, allowed a preliminar test of the performance of MRF in a number of different clinical scenarios. Importantly, MRF synthetic images gave us useful information that could be potentially used to make diagnosis. In addition, parameter maps gave a visible quantitative assessment of signal alteration, which could be followed up.
The underestimation of the number of nodules of heterotopic grey matter and of the extension of gliosis was related to the small dimensions of the lesions. With a more accurate gradient trajectory mapping [2] and B0 correction [3], combined with an optimised acquisition trajectory achieving a higher resolution, it may be possible to further increase the anatomical detail of MRF images.
Conclusion
The MRF ability to produce a number of contrasts in a single, fast and movement-insensitive whole brain acquisition is a promising tool in patients unable to remain still for a standard full exam, especially, but not uniquely, in the paediatric population.The application of MRF has a great potential to address unmet needs in paediatric radiology, and could ideally substitute exams under sedation in a number of cases.Acknowledgements
No acknowledgement found.References
[1] Jiang Y, Ma D, Seiberlich N, Gulani V, Griswold MA. MR fingerprinting using fast imaging with steady state precession (FISP) with spiral readout. Magn Reson Med. 2015 Dec;74(6):1621-31
[2] Tan H Meyer CH Estimation of k-space trajectories in spiral MRI. Magn Reson Med. 2009 Jun;61(6):1396-404. doi: 10.1002/mrm.21813.
[3] Smith TB, Nayak KS. Automatic off-resonance correction in spiral imaging with piecewise linear autofocus. Magn Reson Med. 2013 Jan;69(1):82-90. doi: 10.1002/mrm.24230. Epub 2012 Mar 27.
Figures