4552
Hippocampal stiffness in mesial temporal sclerosis epilepsy measured by MR elastography: Initial results1Biomedical Engineering, University of Delaware, Newark, DE, United States, 2Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL, 3College of Health Sciences, University of Delaware, Newark, DE, United States, 4Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 5Carle Neuroscience Institute, Carle Foundation Hospital, Urbana, IL, United States, 6Department of Molecular and Integrative Physiology, Univerity of Illinois Urbana-Champaign, Urbana, IL, United States
Synopsis
Mesial temporal sclerosis (MTS), or hippocampal sclerosis, is the most common form of temporal lobe epilepsy and can be effectively treated by surgery if it is able to be reliably detected. In this work we examine whether hippocampal stiffness measured by magnetic resonance elastography (MRE) is sensitive to MTS. In our preliminary sample of five patients with MTS and seven controls, we found the hippocampus to be softer bilaterally in MTS (-13.4% and -15.2% differences for affected and unaffected sides). This preliminary evidence suggests MRE may provide highly sensitive markers that could aid the diagnosis and treatment of MTS.
Introduction
The most common form of temporal lobe epilepsy is caused by mesial temporal sclerosis (MTS), or hippocampal sclerosis, where the hippocampus is the epileptogenetic source1. The most successful treatment for MTS epilepsy is hippocampal resection2; however, the lack of consistent imaging evidence from PET and MRI, including hippocampal volume, has limited the early and sensitive detection of MTS and use of surgical treatment options3. Magnetic resonance elastography (MRE) is a novel imaging contrast based on tissue viscoelastic properties4 that may have utility in characterizing MTS given large differences in brain mechanical properties in other neurodegenerative conditions5-7. Additionally, the use of MRE in MTS may be aided by the development of high-resolution methods capable of capturing hippocampal properties8,9. In this initial study we characterize hippocampal stiffness in patients with MTS and compare with healthy control subjects.Methods
Subjects: Five patients diagnosed with MTS were included in this study (4/1 female/male; ages: 21-58). Diagnosis was based on EEG, PET, and T2-FLAIR enhancement as part of clinical care. Seven age- and gender-matched control subjects without MTS or other epilepsy were also included (6/1 female/male; ages 26-60).
Imaging: All scanning was performed using a Siemens 3T Trio scanner. The imaging battery included MRE, T1-weighted MPRAGE, and T2-FLAIR. MRE displacement data was acquired using a 3D multislab, multishot spiral sequence10 with high spatial resolution (1.6x1.6x1.6 mm3 isotropic voxels). Vibrations were generated at 50 Hz using a pneumatic driver with soft, pillow-like actuator under the head (Resoundant, Inc., Rochester, MN). Imaging parameters included: TR/TE=1800/73 ms; 240x240x96 mm FOV; 150x150x60 matrix.
Stiffness Estimation: The MRE images were processed to create stiffness measures using the nonlinear inversion (NLI) algorithm11, which returns the viscoelastic complex shear modulus (G=G’+G’’). From that we calculate the viscoelastic shear stiffness as µ=2|G|2/(G’+G)4. We examined 2 ROI's: to (1) the hippocampus and (2) the temporal lobe (TL). (1) Hippocampal stiffness measures were generated using Freesurfer segmentations12 registered to MRE data, then inverted using soft prior regularization (SPR13). This procedure has been used to generate reliable hippocampal MRE measures previously8,9. (2) TL stiffness measures were generated using a lobar atlas14 registered to the MRE data. Temporal lobe masks included gray and white matter, but excluded CSF, through use of FAST segmentations in FSL15.
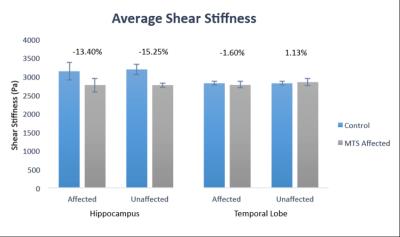
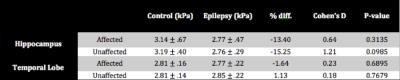
Analysis: Both hippocampus and TL results were analyzed unilaterally depending on seizure side: affected (epileptogenetic) and unaffected. The left side was affected in all MTS patients, and we chose the left side as the “affected” side in controls for comparison. One patient had bilateral MTS and this data was included only in the analysis of affected side (chosen as left). Each measure and side were compared between groups using a t-test and we report percent differences from control and Cohen’s d effect sizes.
Results
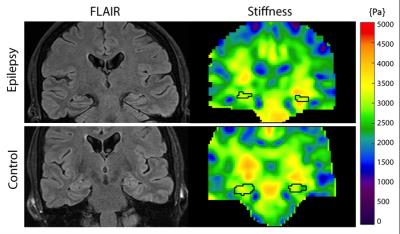
Example stiffness maps for an MTS patient and healthy control are presented in Figure 1, with outlines of hippocampi. Figure 2 presents average properties for both affected and unaffected hippocampi from patients and controls, and these results are collected in Table 1. The average hippocampal shear stiffness is lower for the MTS patients than the healthy controls with differences of -13.4% and -15.2% in the affected and unaffected sides, respectively. These differences were not statistically significant, in part due to the small number of subjects currently enrolled in the study. However, large effect sizes (0.64 and 1.21) suggest a high sensitivity of hippocampal stiffness to the presence of MTS. Further, this effect appears to be confined to the hippocampus, as there was minimal effect in the overall TL (-1.6% and 1.1%).Discussion and Conclusions
Our data indicates that MRE is sensitive to hippocampal degradation that occurs in MTS epilepsy. Here, we show that the hippocampus is softer both on the affected side, but also on the unaffected side, supporting that there is also tissue damage occurring contralateral to seizure onset. This damage is centralized to the hippocampi, as the TL on either side do not appear to exhibit any measurable degradation. Future work will look to expand on these results by examining more patients and controls, in order to determine if these effects are significant, and further investigate the role of disease progression in these results to determine the potential role for MRE in the diagnosis and treatment of MTS.Acknowledgements
This work was supported by the Carle Neuroscience Institute of Carle Foundation Hospital, the Biomedical Imaging Center of the Beckman Institute at University of Illinois, and by a grant from the ACCEL Program of the Delaware Center for Translational Research. The ACCEL Program is funded by NIGMS IDeA Grant U54-GM104941.References
[1] Engel J. (2001): Mesial Temporal Lobe Epilepsy: What Have We Learned? Neuroscientist; 7:340–352
[2] Wiebe S, Blume WT, et al. (2001): A Randomized, Controlled Trial of Surgery for Temporal-Lobe Epilepsy. New Engl J Med; 345:311–318.
[3] Engel J, McDermott MP, et al. (2012): Early Surgical Therapy for Drug-Resistant Temporal Lobe Epilepsy: a Randomized Trial. JAMA; 307:922–930.
[4] Manduca A, Oliphant TE, et al. (2001): Magnetic Resonance Elastography: Non-Invasive Mapping of Tissue Elasticity. Med Image Anal 5:237–254.
[5] Murphy MC, Jones DT, et al. (2016): Regional brain stiffness changes across the Alzheimer's disease spectrum. NeuroImage: Clin; 10:283-290.
[6] Streitberger K-J, Sack I, et al. (2012): Brain Viscoelasticity Alteration in Chronic-Progressive Multiple Sclerosis. PLoS One; 7:e29888.
[7] Lipp A, Trbojevic R, et al. (2013): Cerebral magnetic resonance elastography in supranuclear palsy and idiopathic Parkinson's disease. NeuroImage Clin; 3:381–387.
[8] Johnson CL, Schwarb H, et al. (2016): Viscoelasticity of subcortical gray matter structures." Hum Brain Mapp; 37:4221-4233.
[9] Schwarb H, Johnson CL, et al. (2016): Medial temporal lobe viscoelasticity and relational memory performance. NeuroImage; 132: 534-541.
[10] Johnson CL, Holtrop JL, et al. (2014): 3D multislab, multishot acquisition for fast, wholebrain MR elastography with high signal-to-noise efficiency. Magn Reson Med; 71:477–485.
[11] McGarry MDJ, Van Houten EEW, et al. (2012): Multiresolution MR elastography using nonlinear inversion. Med Phys; 39:6388–6396.
[12] Fischl B, Salat DH, et al. (2002): Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron; 33:341–355.
[13] McGarry MDJ, Johnson CL, et al. (2013): Including spatial information in nonlinear inversion MR elastography using soft prior regularization. IEEE T Med Imaging; 32:1901–1909.
[14] Maldjian JA, Laurienti PJ, et al. (2003): An Automated Method for Neuroanatomic and Cytoarchitectonic Atlas-Based Interrogation of fMRI Data Sets. NeuroImage; 19:1233–1239.
[15] Zhang Y, Brady M, et al. (2001): Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE T Med Imaging; 20:45–57.
Figures