4543
Advances in imaging the human brain with inhaled hyperpolarized xenon-129 MRI at 1.5 T1University Of Sheffield, Sheffield, United Kingdom
Synopsis
The feasibility of imaging human brain tissue-perfusion using inhaled hyperpolarized 129Xe magnetic resonance imaging is demonstrated. Enhancement of 129Xe gas polarization and a custom brain RF coil array have together enabled imaging of hyperpolarized 129Xe dissolved in the human brain at 1.5 T with a quality and signal-to-noise hitherto unseen. The images clearly demonstrate the uptake and washout of 129Xe in the brain with time and could provide novel insights into cerebral perfusion and blood brain barrier permeability without the use of intravenous contrast.
Introduction
The feasibility of imaging human brain tissue-perfusion using inhaled hyperpolarized 129Xe magnetic resonance imaging is demonstrated. Upon inhalation, xenon dissolves in the pulmonary vasculature and is transferred to the brain through the systemic blood circulation1. The T1 of xenon dissolved in blood2 is long enough (6~8 s) that inhaled hyperpolarized (HP) 129Xe can be detected when dissolved in the brain tissue, beyond the intact blood brain barrier1. In this study, utilizing a novel RF array coil design and highly polarized 129Xe gas, we demonstrate HP 129Xe MRI as a safe and non-invasive agent for imaging human brain perfusion.Methods
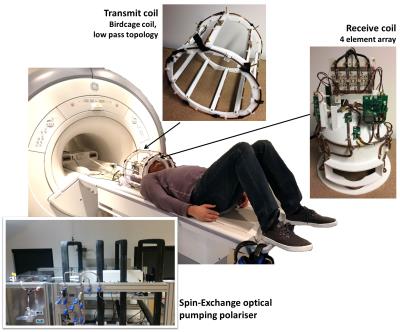
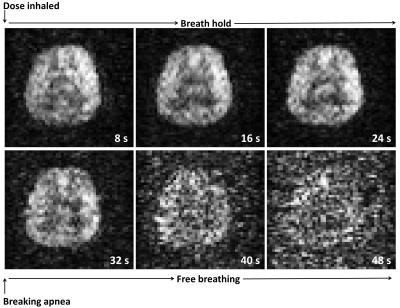
129Xe was hyperpolarized using a home-built spin-exchange optical pumping polarizer which can generate ~35% 129Xe polarization at a production rate of ~1.2 L/hour. In-vivo imaging was performed on a 1.5 T clinical MRI scanner using a home-built 4 element receiver coil array and a transmit birdcage coil of low-pass topology, and the inhaled xenon gas dose was 1 L. The setup is shown in Figure 1. MR imaging parameters were; spoiled gradient echo pulse sequence, center frequency = 198 ppm downfield from the 129Xe gas resonance, echo time = 1.7 ms, repetition time = 34 ms, flip angle = 12.5°, bandwidth = 227 ppm, field of view = 22 cm, slice thickness = 50 mm, acquisition matrix size of 32 x 32 (reconstructed to 48 x 48). Three images were acquired during a single breath hold at 8 s, 16 s and 24 s after the inhalation of the xenon gas dose, and three additional images were subsequently acquired at 32 s, 40 s and 48 s after the volunteer resumed breathing at 24 s. The images acquired at 8 s, 16 s, 24 s and 32 s were averaged and signal-to-noise ratio (SNR) was calculated as a ratio of mean signal to background noise3Results
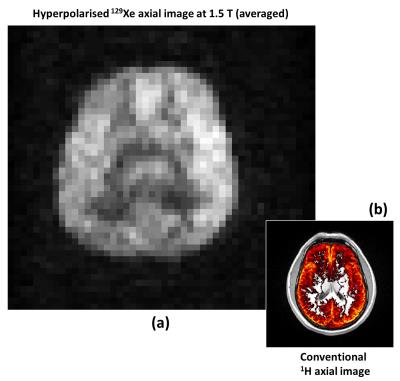
A time-course of in vivo axial MR images of HP 129Xe dissolved in the human brain acquired at 8 s, 16 s, 24 s, 32 s, 40 s and 48 s after inhalation are shown in Figure 2. The average of four images (acquired at 8 s, 16 s, 24 and 32 s) is shown in Figure 3(a). The SNR of averaged image was 30. Figures 3(b) shows a 1H image segmented for gray matter (in color) and superimposed on a 1H image (gray scale) from the same anatomical location as the 129Xe brain image. Figure 3(a) and 3(b) exhibit structural similarities, which confirms previous spectroscopic findings that imaging xenon dissolved in the brain is weighted towards gray matter1.Discussion
The time resolved imaging demonstrates the regional uptake of xenon by the brain tissue and the potential to quantify the washout of xenon from the brain tissues. For the assessment of brain tissue perfusion, the technique has a number of potential advantages over existing clinical methods because by imaging HP 129Xe dissolved in the extravascular tissue compartment, we are directly imaging the perfused tissue rather than imaging the intravascular compartment, e.g. using exogenous iodine or gadolinium-based contrast agents4.
Although previous studies have been conducted to image HP 129Xe dissolved in rat brain tissue, these studies were conducted in the anaesthetized state5,6. It has been observed earlier that cerebral blood flow does not change in rats under a stable-anaesthetized state7, unlike in humans8. Thus, it is neither possible to translate the findings of these earlier studies in rats5,6 into the context of human clinical studies, nor is it practical to perform human studies using the same protocols. In that respect, this study offers many practical advantages as it does not require the subject to be either anaesthetized or to be on respiratory apparatus.
Nevertheless, image resolution and SNR in the present study is limited by the quantity of HP 129Xe that is transported and delivered to the brain tissue and the polarization of 129Xe as it decays through longitudinal relaxation on its journey from the lungs to the brain by the processes of gas phase relaxation in the lungs and dissolved phase relaxation in blood and tissue.
Conclusion
We have demonstrated dissolved 129Xe images from the human brain with a quality hitherto not realized. A number of clinical applications of dissolved xenon brain imaging can be envisaged, such as brain ischemia, stroke, Alzheimer’s diseases, vascular dementia, atherosclerosis, brain tumors, infections and multiple-sclerosis. The high solubility of xenon in lipids also opens up the diagnostic potential for evaluation of brain tumors. These clinical applications are the scope of future-work.Acknowledgements
This work was funded by the Engineering and Physical Sciences Research Council (EPSRC), National Institute for Health Research (NIHR) and Medical Research Council (MRC). This report is independent research supported by an NIHR Research Professorship.References
1. Rao M, Stewart NJ, Norquay G, Griffiths PD, Wild JM. High resolution spectroscopy and chemical shift imaging of hyperpolarized 129Xe dissolved in the human brain in vivo at 1.5 tesla. Magnetic Resonance in Medicine 2016: DOI 10.1002/mrm.26241.
2. Wolber J, Cherubini A, Leach MO, Bifone A. Hyperpolarized 129Xe NMR as a probe for blood oxygenation. Magnetic Resonance in Medicine 2000;43(4):491-496.
3. Collins CM, Smith MB. Signal-to-noise ratio and absorbed power as functions of main magnetic field strength, and definition of "90°" RF pulse for the head in the birdcage coil. Magnetic Resonance in Medicine 2001;45(4):684-691.
4. Wintermark M, Sesay M, Barbier E, Borbely K, Dillon WP, Eastwood JD, Glenn TC, Grandin CB, Pedraza S, Soustiel JF, Nariai T, Zaharchuk G, Caille JM, Dousset V, Yonas H. Comparative overview of brain perfusion imaging techniques. Stroke 2005;36(9):e83-99.
5. Mazzanti ML, Walvick RP, Zhou X, Sun Y, Shah N, Mansour J, Gereige J, Albert MS. Distribution of hyperpolarized xenon in the brain following sensory stimulation: preliminary MRI findings. PloS one 2011;6(7):e21607.
6. Zhou X, Sun Y, Mazzanti M, Henninger N, Mansour J, Fisher M, Albert M. MRI of stroke using hyperpolarized 129Xe. NMR Biomed 2011;24(2):170-175.
7. Frietsch T, Bogdanski R, Blobner M, Werner C, Kuschinsky W, Waschke KF. Effects of xenon on cerebral flood flow and cerebral glucose utilization in rats. Anesthesiology 2001;94(2):290-297.
8. Laitio RM, Kaisti KK, Langsjo JW, Aalto S, Salmi E, Maksimow A, Aantaa R, Oikonen V, Sipila H, Parkkola R, Scheinin H. Effects of xenon anesthesia on cerebral blood flow in humans - A positron emission tomograpby study. Anesthesiology 2007;106(6):1128-1133.
Figures