4534
TBI Patients with Cerebral Microhemorrhage Exhibit Increased Magnetic Susceptibility in the Cerebral Hemispheres, but Reduced Magnetic Susceptibility in the Basal Ganglia1National Intrepid Center of Excellence, Walter Reed National Military Medical Center, Bethesda, MD, United States, 2The NorthTide Group, LLC, Dulles, VA, United States
Synopsis
Magnetic susceptibilities of the basal ganglia, as well as the right and left cerebral hemispheres of TBI patients with cerebral microhemorrhage (CMH) were analyzed. Compared to patients without CMH and controls, patients with CMH demonstrated increased magnetic susceptibility in both the left and right hemispheres but decreased magnetic susceptibility in the basal ganglia. This finding suggests disrupted brain iron hemostatsis due to CMH in the chronic phase of TBI.
Introduction
Cerebral microhemorrhage (CMH) is a common finding in patients with traumatic brain injury (TBI)1,2. Blood products in CMH undergo a complex degradation process from oxyhemoglobin to deoxyhemoglobin and finally hemosiderin over a period of months. Previous investigation of CMH in military TBI patients using quantitative susceptibility mapping (QSM) revealed fewer numbers of CMH at follow-up compared to baseline3. Furthermore, both the volume and the magnetic susceptibility of CMH decreased from baseline to follow-up suggesting hemosiderin products undergo continued, subtle evolution. However, it is unknown if the deposition of the blood products affects the brain iron metabolism. Iron content in the brain is a tightly regulated process with excess iron being toxic and associated with multiple neurologic disorders. In this study, magnetic susceptibilities of the basal ganglia, as well as the right and left cerebral hemispheres of patients with CMH were analyzed to investigate the potential impact of CMH on brain iron deposition.Methods
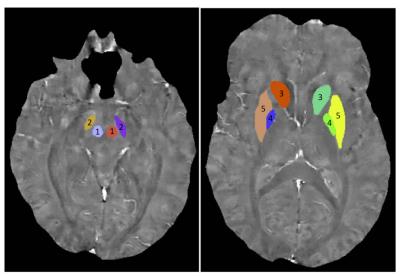
12 military TBI patients (all male) with CMH, 33 TBI patients (6 female) without CMH and 11 non-TBI controls (all male) were included. The age of the subjects was 30 ± 8 years for patients with CMH, 28 ± 10 years for patients without CMH and 31 ± 6 years for the controls. The time since injury of the subjects was 163 ± 108 days for patients with CMH and 135 ± 88 days for patients without CMH. Images were acquired on a 3T scanner with a 32-channel phased array head coil. Structural T1 images were acquired with the 3D BRAVO sequence: TR/TE = 6.7/2.5 ms, FA = 12°, voxel size = 0.5 × 0.5 × 0.6 cm3. The multi-echo images used for QSM were acquired using a 3D flow-compensated multi-echo gradient-echo sequence with TR = 45 ms, a total of 5 echoes, TE0 = 13 ms, echo-spacing = 6 ms, FA = 20°, FOV = 24 × 24 cm2, 512 × 256 matrix, 88 slices at 1.5 mm thickness. QSM images were calculated using the morphology-enabled dipole inversion (MEDI) software4. Freesurfer segmentation was performed on the T1 images. The right and left cerebral hemispheres were extracted from the Freesurfer segmentation. A threshold of 40 - 200 ppb was set for the mean susceptibility of the left and right hemispheres to highlight regions with iron deposition and vascular structures. Five basal ganglia regions that are known to be rich in iron: red nucleus (RN), substantia nigra (STN), caudate, pallidum and putamen, were manually segmented on the QSM images (Figure 1).Results
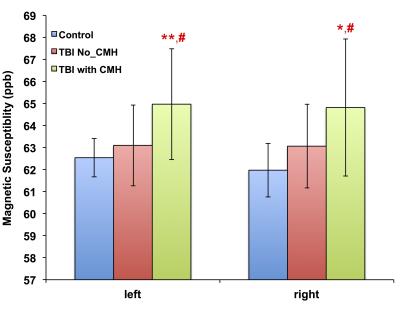
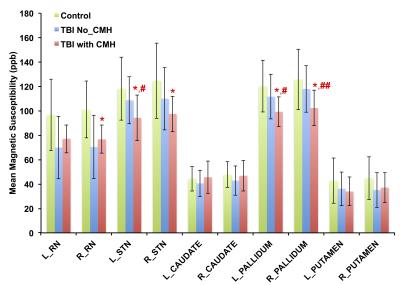
Patients with CMH exhibit significantly higher magnetic susceptibility compared to controls and patients without CMH (Figure 2) in the left hemisphere: 65.0 ± 2.5 ppb vs. 62.5 ± 0.9 ppb in controls (p = 0.01, d = 1.28) and 63.1 ± 1.8 ppb in patients without CMH (p = 0.03, d = 0.84) and in the right hemisphere: 64.8 ± 3.1 ppb vs. 62.0 ± 1.2 ppb in controls (p = 0.01, d = 1.20). However, patients with CMH demonstrate decreased magnetic susceptibility compared to controls and patients without CMH in the right red nucleus: 76.7 ± 11.5 ppb vs. 101.0 ± 23.2 ppb in controls (p = 0.01, d = 1.20), in the left substantia nigra: 94.3 ± 18.7 vs. 118.2 ± 25.9 ppb in controls (p = 0.02, d = 0.92) and 108.6 ± 19.2 ppb in patients without CMH (p = 0.04, d = 0.73); in the right substantia nigra: 97.4 ± 14.6 ppb vs. 124.7 ± 30.9 ppb in controls (p = 0.02, d = 1.12); in the left Pallidum: 99.2 ± 12.2 ppb vs. 120.2 ± 21.2 ppb in controls (p = 0.01, d = 1.13) and 111.5 ± 18.4 ppb in patients without CMH (p = 0.01, d = 0.80), and in the right Pallidum: 102.2 ± 14.5 ppb vs. 125.6 ± 24.8 in controls (p = 0.01, d = 1.22) and 117.8 ± 9.0 ppb in patients without CMH (p = 0.01, d = 0.95) (Figure 3).Conclusion
Compared to patients without CMH and controls, patients with CMH demonstrated increased magnetic susceptibility in both the left and right cerebral hemispheres but decreased magnetic susceptibility in the basal ganglia. This finding suggests disrupted brain iron hemostatsis due to CMH in the chronic phase of TBI and may be a potential biomarker of injury.Disclaimer
The views expressed in this abstract are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or the U.S. Government.Acknowledgements
We would like to acknowledge our grant support: #13129004 "Advanced imaging acquisition and data analysis for a military TBI Neuroimaging database" from USAMRMC.References
1.Report to Congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. National Center for Injury Prevention and Control Centers for Disease Control and Prevention. 2003.
2. Marion DW, Darby J, Yonas H. Acute regional cerebral blood flow changes caused by seere head injuries, J Neurosurg 1991;74:407-14.
3. Liu W, Soderlund K, Yeh P-H et al. Characterize Cerebral Microhemorrhages in Patients with Chronic Traumatic Brain Injury, Radiology, 2016; 278:536-45.
4. Liu T, Liu J, de RochefortL et al., Morphology enabled dipole inversion (MEDI) from a single-angle acquisition: comparison with COSMOS in human brain imaging. Magn Reson Med 2011; 66:777-783.
Figures