4531
Identifying potential sites of brain injury in an individual concussed football player using a normative database based on diffusion kurtosis imaging1Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, United States, 2Department of Biophysics, Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
Mild traumatic brain injury (mTBI) is a prevalent health problem, especially in full-contact sports1, 6. Despite its prevalence, there is still a lack of reliable, unbiased biomarkers of brain injury and recovery following mTBI. Diffusion weighted MRI techniques have gained attention recently in studies of mTBI. Diffusion kurtosis tensor imaging (DKTI) is an extension of the conventional DTI, which estimates non-Gaussianity of bulk diffusion in each voxel. Our studies indicated that DKTI might be potential biomarker to detect subtle changes in brain tissues5. Here, we introduce a workflow to detect sites of brain injury in an individual concussed subject.
Purpose
To this date, most mTBI studies focused on group-based comparisons, testing differences between concussed and control subjects. Such studies help understand which brain regions are typically affected by concussion so that this type of injury can be better understood and managed. However, clinicians need diagnostic tools to identify sites of injury in individual patients. Therefore, the goal here was to develop a workflow to identify which brain regions might be injured in an individual mTBI patient. For this purpose, a normative database of DKTI metrics was built using images from a carefully matched control group. Then, DKTI maps from a concussed athlete was registered and compared against this database to mark regions that deviate significantly from the normal range of values.Methods
DKTI data for normative database were acquired from 26 high school and college football players with no history of concussion (at least none in the last year). Also, anyone with a history of moderate or severe TBI or other medical conditions known to cause cognitive dysfunction (e.g., epilepsy, stroke) were excluded. Images were also reviewed by a neuroradiologist for incidental findings. Injured subjects underwent the same procedures. The study was approved by the IRB and written consents were obtained from participants. Subjects were administered the SCAT32 postconcussion symptom checklist preseason, within 24 hours of injury, and 8 days post-injury. DKTI data were acquired within 24 hours of injury, and then 8 days after injury. Single-shot SE-EPI sequence was used with 3mm-isotropic voxels, b=1000s/mm2 and 2000s/mm2, four b=0 (reference images) and 30 diffusion directions for each b-value. Images were processed through software developed in-house to estimate DKI tensors based on the algorithm published earlier4. Fractional anisotropy, mean, axial, and radial diffusivity (FA, MD, Dax, Drad) and mean, axial, and radial kurtosis (MK, Kax, Krad) maps were generated for each participant.
Generation of normative data: DKTI maps from the first scans of the control group were registered to the MNI space. For accurate registration, average of b=0 images from each subject was first registered to his T1 image using 6-parameter affine transformation. Then his T1 image was normalized to MNI152_T1 template using FSL’s affine and nonlinear registration tools3. Then, these registrations were applied to the DKTI maps to transform them to the MNI space. Once all DKTI maps from control subjects were transformed to the MNI space, the mean and standard deviation across subjects were calculated for each voxel and saved for analysis. This procedure was repeated separately for each DKTI map. To test the images of a concussed subject against this normative database, his image was first transformed to MNI space using the same registration procedures. Then, the measurement yvox at each voxel of the image was converted to a t-score using the following formula:
tvox=(yvox–mvox)/(svox*sqrt(1+1/26)).
Here mvox and svox are the voxel-wise mean and standard deviation derived from the normative data. T-score maps were converted to p values and corrected for multiple comparisons using False Discovery Rate (FDR). Any voxel that was significant at p<0.05 (FDR-corrected) was marked on the T1 image of the subject in standard space.
Results
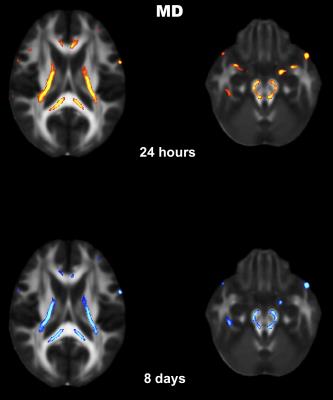
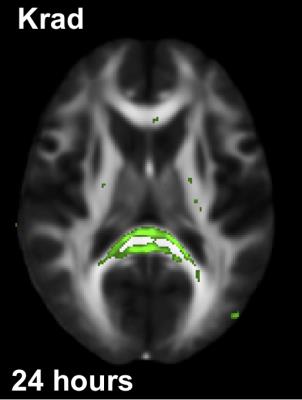
Fig.1 shows the brain regions of a concussed subject (SCAT3=62 at the 24-hour time point), whose MD values were significantly higher than the normative values. Red and blue overlays show differences at the 24 hour and the 8-day time points, respectively. At both time points, the base of the cortico-spinal tracts, splenium and posterior limb of internal capsule were bilaterally affected. Fig.2. shows brain regions of the same subject where Krad values were significantly lower than the normative database mainly in the splenium. Krad was significantly different only at the 24-hour scans.Discussion and Conclusion
Here we introduced a feasible workflow to assess the site and extent of potential brain injury in a subject with concussion. Although the results presented in Fig.1 and Fig.2 demonstrate the feasibility of the proposed method, the workflow needs to be refined with a larger database of normal controls and extensively validated. Since the precision of registration is critical for this type of comparisons, we visually inspected the registered images for accuracy. We will explore if further improvements in registration accuracy could be achieved using diffeomorphic registration methods.Acknowledgements
This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (8UL1TR000055 and 1UL1-RR031973-01), the US Army Medical Research and Materiel Command (W81XWH-12-1-0004), and the NFL-GE Head Health Challenge I.References
1. Giza, C.C. and Hovda, D.A., The new neurometabolic cascade of concussion., Neurosurgery, 75 Suppl 4 (2014) S24-33.
2. Guskiewicz, K.M. et al., Evidence-based approach to revising the SCAT2: introducing the SCAT3., Br J Sports Med, 47 (2013) 289-293.
3. Jenkinson, M. et al., FSL., Neuroimage, 62 (2012) 782-790.
4. Jensen, J.H. et al., Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging., Magn Reson Med, 53 (2005) 1432-1440.
5. Lancaster, M.A. et al., Acute white matter changes following sport-related concussion: A serial diffusion tensor and diffusion kurtosis tensor imaging study., Hum Brain Mapp, 37 (2016) 3821-3834.
6. McCrory, P. et al., Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012., Br J Sports Med, 47 (2013) 250-258.
Figures