4529
Quantitative Susceptibility Mapping of Hockey Players After Mild Traumatic Brain Injury1Pediatrics, University of British Columbia, Vancouver, BC, Canada, 2University of British Columbia, Vancouver, BC, Canada, 3Radiology, University of British Columbia, Vancouver, BC, Canada, 4Division of Sports Medicine, University of British Columbia, Vancouver, BC, Canada, 5Pediatrics, University of British Columbia, BC, Canada
Synopsis
We followed 45 varsity hockey players during one season of play, and scanned all players at the beginning of the season. 11 players received a concussion, and were scanned within 72hrs post-concussion, and then again after 2 weeks and 2 months. Quantitative Susceptibility Maps were created from the multi-echo 3D gradient-echo data, and susceptibility values were measured in deep grey matter (caudate, pallidum, putamen, and thalamus) and frontal and posterior WM in the corpus callosum (genu and splenium). A linear mixed-effect model analysis of the regions of interest revealed no significant changes over time compared to baseline.
Introduction
Mild
traumatic brain injury (mTBI; concussion) is a very
common form of brain damage sustained through sports, traffic
accidents, blasts, and/or slips/trips/falls, yet remains poorly
understood. There is currently no gold standard for its diagnosis.
The
symptoms vary from small, such as no symptoms, headaches and/or
imbalance, to unconsciousness (under 30mins) and cognitive or
behavioural deficits [1]. The
pathophysiology of concussions include the impact of the brain with
the skull, resulting in cerebral contusions, the stretching of and
damage to axons, and often times a 'contrecoup' to the opposite side
of the head [2]. Secondary
processes further broaden the spectrum of possible symptoms. Animal studies have shown that mTBI can create oxidative stress and axonal damage in the absence
of gross focal lesions [3,4]. Iron accumulation in the pathology of TBI has been found in the brains of mice after a controlled cortical impact injury [5]. To get a
better understanding of mTBI, we used Quantitative Susceptibility
Mapping (QSM) to measure magnetic susceptibility changes in the brains of
ice-hockey players after a concussion. Eleven of forty-five varsity hockey players received a concussion during one season of play. These subjects underwent
a pre-season baseline scan with follow-up scans at three-days, two-weeks and two-months post-concussion.
Methods
Subjects:
Forty-five ice hockey-players underwent a pre-season baseline scan and took part in follow-up scans (72 hours, 2 weeks, 2 months) if they received a concussion during play (assessed using Sport Concussion Assessment Tool 2 by a physician). 11 players received a concussion during play (5 males, 6 females). Their images were acquired with a 3T Philips Achieva scanner.
Data Acquisition:
Acquired MRI images were: 3D-sagittal T1-weighted image (TR=8.1ms, TE=3.7ms, flip angle=6°, voxel size= 1x1x1mm^3, acquisition matrix= 256x256x160, field of view= 256x256x160mm^3) and a multi-echo SWI with a 3D gradient echo (TR=36ms, TE=6,12,18,24,30ms, flip angle=17°, acquisition matrix=440x222x64, FOV=220x166x128mm^3, voxel size= 0.5x0.5x1mm^3).
Image Analysis:
All SWI images were post-processed as QSM images using in-house MATLAB code [6]. FSL (FMRIB Software Library, Oxford, United Kingdom), was used for display, brain extraction, segmentation and registration of the regions of interest. For the DGM structures, FSL FIRST was used to segment ROIs, whereas the ICBM-DTI-81 WM labeled atlas from the Johns Hopkins University [7] was used for the WM ROIs.
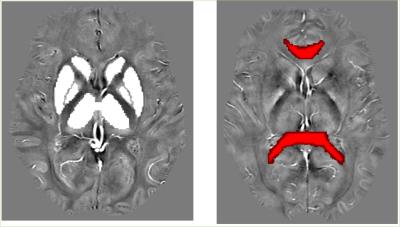
Sample segmentation and atlas registration are shown in Fig 1.
Statistics:
Statistical analysis was performed with MATLB and Statistics Toolbox Release 2016a (The MathWorks, Inc., Natick, Massachusetts, United States). Each region was processed separately with the use of a linear mixed-effect model. A multiple comparison corrected p-value lower than 0.05 indicated a significant correlation.
Results
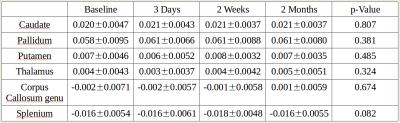
All 11 concussed subjects (21.18 ± 1.66 years old) had successfully undergone a baseline scan, with 8, 10 and 9 scans acquired within 72hours, two weeks, and two months post-concussion, respectively. Statistical analysis showed neither a significant correlation between a mTBI and DGM susceptibility value changes nor in the white matter regions (p-value > 0.05 (uncorrected)). (Fig 2)
Discussion
We found no significant magnetic susceptibility changes at 3 days, 2 weeks, and 2 months post-concussion in the DGM (caudate, pallidum, putamen, and thalamus) or CC WM (genu and splenium).
Previous studies, using similar MRI methods, have found increased magnetic susceptibility levels in thalamus [5,8,9], globus pallidus [8], caudate [9], lenticular nucleus [9], hippocampus [9], right substantia nigra [9], red nucleus [9], splenium [9], and internal capsule [5]. These findings were often much later (18months [8], 19 months [9], and 1-2 months in mice [5]) than our own time-points, suggesting a possible delay in iron accumulation post-concussion before being detectable using MRI.
This is the first study to measure magnetic susceptibility both before and soon after concussion. Previously published findings from the same cohort showed reduced fractional anisotropy in the genu (but no changes in DGM) [10], and reduced myelin water fraction (MWF) in splenium [11]. Thus, although we can detect changes in microarchitecture and myelin levels after sport-related mTBI, secondary accumulation of iron, at 3 days, 2 weeks, or 2 months, may not have yet occurred or accumulated enough to be detectable through 3T QSM. Alternatively, lack of change in splenium susceptibility may be interpreted as no reduction of myelin content in these areas, which is in agreement with our finding of recovery at 2 months with MWF [11]. This suggests that myelin changes, but is not reduced.
Acknowledgements
Author contributions: A.R., J.T. and D.L. designed the study. A.R. and D.L. designed the imaging protocol. M.J. and S.D. collected data and helped coordinate the study. A.P. performed data analysis under the supervision of A.M.W. A.P. and A.M.W. wrote the manuscript. C.K. wrote the in-house MATLAB QSM algorithm software. Competing interests: The authors declare that they have no competing interests.
Funding: Canada Research Chairs, CFRI postdocoral fellowship, DAAD RISE program, and London Drugs Award.
References
1. Mechtler LL, Shastri KK, Crutchfield KE. Advanced neuroimaging of mild traumatic brain injury. Neurol Clin. 2014 Feb;32(1):31–58.
2. Smith DH, Meaney DF. Axonal Damage in Traumatic Brain Injury. The Neuroscientist. 2000 Dec 1;6(6):483–95.
3. Bakay L, Lee JC, Lee GC, et al. Experimental cerebral concussion. Part 1: an electron microscopic study. J Neurosurg 1977;47:525–31 MedlineGoogle Scholar
4. Jane JA, Steward O, Gennarelli TA. Axonal degeneration induced by experimental noninvasive minor head injury. J Neurosurg 1985;62:96–100
5. Onyszchuk G, LeVine SM, Brooks WM, Berman NEJ. Post-acute pathological changes in the thalamus and internal capsule in aged mice following controlled cortical impact injury: a magnetic resonance imaging, iron histochemical, and glial immunohistochemical study. Neurosci Lett. 2009 Mar 13;452(2):204–8.
6. Kames, Wiggermann, Rauscher, Rapid Two-Step Dipole Inversion for QSM, Magn Reson Med, in revision.: QSM were computed from the SWI scan using rapid two step dipole inversion. In revision
7. Mori S, Crain BJ. MRI atlas of human white matter [Internet]. Amsterdam; Boston: Elsevier; 2005 [cited 2016 Oct 24]. Available from: http://public.eblib.com/choice/publicfullrecord.aspx?p=269986
8. Raz E, Jensen JH, Ge Y, Babb JS, Miles L, Reaume J, et al. Brain iron quantification in mild traumatic brain injury: a magnetic field correlation study. AJNR Am J Neuroradiol. 2011 Dec;32(10):1851–6.
9. Lu L, Cao H, Wei X, Li Y, Li W. Iron Deposition Is Positively Related to Cognitive Impairment in Patients with Chronic Mild Traumatic Brain Injury: Assessment with Susceptibility Weighted Imaging. BioMed Res Int. 2015;2015:470676.
10. Weber AM, Jarrett M, Hernández-Torres E, Dadachanji S, Li DKB, Taunton J, et al. Diffusion Imaging Reveals White Matter Damage in Ice Hockey Players for Up To Two Months Post-Concussion. In review.
11. Wright AD, Jarrett M, Vavasour I, Shahinfard E, Kolind S, van Donkelaar P, et al. Myelin Water Fraction Is Transiently Reduced after a Single Mild Traumatic Brain Injury – A Prospective Cohort Study in Collegiate Hockey Players. de Castro F, editor. PLOS ONE. 2016 Feb 25;11(2):e0150215.